Pharmacodynamic Correlates of Linezolid Activity and Toxicity in Murine Models of Tuberculosis
- PMID: 31993638
- PMCID: PMC8176636
- DOI: 10.1093/infdis/jiaa016
Pharmacodynamic Correlates of Linezolid Activity and Toxicity in Murine Models of Tuberculosis
Abstract
Background: Linezolid (LZD) is bactericidal against Mycobacterium tuberculosis, but it has treatment-limiting toxicities. A better understanding of exposure-response relationships governing LZD efficacy and toxicity will inform dosing strategies. Because in vitro monotherapy studies yielded conflicting results, we explored LZD pharmacokinetic/pharmacodynamic (PK/PD) relationships in vivo against actively and nonactively multiplying bacteria, including in combination with pretomanid.
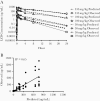
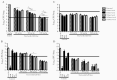
Methods: Linezolid multidose pharmacokinetics were modeled in mice. Dose-fractionation studies were performed in acute (net bacterial growth) and chronic (no net growth) infection models. In acute models, LZD was administered alone or with bacteriostatic or bactericidal pretomanid doses. Correlations between PK/PD parameters and lung colony-forming units (CFUs) and complete blood counts were assessed.
Results: Overall, time above minimum inhibitory concentration (T>MIC) correlated best with CFU decline. However, in growth-constrained models (ie, chronic infection, coadministration with pretomanid 50 mg/kg per day), area under the concentration-time curve over MIC (AUC/MIC) had similar explanatory power. Red blood cell counts correlated strongly with LZD minimum concentration (Cmin).
Conclusions: Although T>MIC was the most consistent correlate of efficacy, AUC/MIC was equally predictive when bacterial multiplication was constrained by host immunity or pretomanid. In effective combination regimens, administering the same total LZD dose less frequently may be equally effective and cause less Cmin-dependent toxicity.
Keywords: linezolid; mouse; pharmacodynamics; pharmacokinetics; tuberculosis.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- World Health Organization. Global tuberculosis report 2018. Geneva: World Health Organization; 2018.
-
- World Health Organization. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). Geneva: WHO, World Health Organization; 2018.
-
- Conradie F, Diacon A, Howell P, et al. . Sustained high rate of successful treatment outcomes: interim results of 75 patients in the Nix-TB clinical study of pretomanid, bedaquiline and linezolid. The Hague, Netherlands: The Union World Conference on Lung Health; 2018.
-
- Abbate E, Vescovo M, Natiello M, et al. . Successful alternative treatment of extensively drug-resistant tuberculosis in Argentina with a combination of linezolid, moxifloxacin and thioridazine. J Antimicrob Chemother 2019; 67:473–7. - PubMed
-
- Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest 2008; 134:187–92. - PubMed

