First-In-Human Experience With Integration of Wireless Intracranial Pressure Monitoring Device Within a Customized Cranial Implant
- PMID: 31993644
- PMCID: PMC7594174
- DOI: 10.1093/ons/opz431
First-In-Human Experience With Integration of Wireless Intracranial Pressure Monitoring Device Within a Customized Cranial Implant
Abstract
Background: Decompressive craniectomy is a lifesaving treatment for intractable intracranial hypertension. For patients who survive, a second surgery for cranial reconstruction (cranioplasty) is required. The effect of cranioplasty on intracranial pressure (ICP) is unknown.
Objective: To integrate the recently Food and Drug Administration-approved, fully implantable, noninvasive ICP sensor within a customized cranial implant (CCI) for postoperative monitoring in patients at high risk for intracranial hypertension.
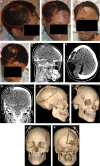
Methods: A 16-yr-old female presented for cranioplasty 4-mo after decompressive hemicraniectomy for craniocerebral gunshot wound. Given the persistent transcranial herniation with concomitant subdural hygroma, there was concern for intracranial hypertension following cranioplasty. Thus, cranial reconstruction was performed utilizing a CCI with an integrated wireless ICP sensor, and noninvasive postoperative monitoring was performed.
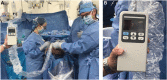
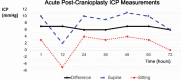
Results: Intermittent ICP measurements were obtained twice daily using a wireless, handheld monitor. The ICP ranged from 2 to 10 mmHg in the supine position and from -5 to 4 mmHg in the sitting position. Interestingly, an average of 7 mmHg difference was consistently noted between the sitting and supine measurements.
Conclusion: This first-in-human experience demonstrates several notable findings, including (1) newfound safety and efficacy of integrating a wireless ICP sensor within a CCI for perioperative neuromonitoring; (2) proven restoration of normal ICP postcranioplasty despite severe preoperative transcranial herniation; and (3) proven restoration of postural ICP adaptations following cranioplasty. To the best of our knowledge, this is the first case demonstrating these intriguing findings with the potential to fundamentally alter the paradigm of cranial reconstruction.
Keywords: Cranial; Cranioplasty; ICP; Implant; Intracranial; Monitoring; Neurotechnology; Pressure; Skull.
© Congress of Neurological Surgeons 2020.
Figures



References
-
- Dewan MC, Rattani A, Gupta Set al.. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130(4):1039-1408. - PubMed
-
- Hutchinson PJ, Kolias AG, Timofeev ISet al.. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375(12):1119-1130. - PubMed
-
- Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006(1):CD003983. - PubMed
-
- Badri S, Chen J, Barber Jet al.. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012;38(11):1800-1809. - PubMed
-
- Timofeev I, Santarius T, Kolias AG, Hutchinson PJ. Decompressive craniectomy - operative technique and perioperative care. Adv Tech Stand Neurosurg. 2012;38:115-136. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

