A Narrative Pharmacological Review of Buprenorphine: A Unique Opioid for the Treatment of Chronic Pain
- PMID: 31994020
- PMCID: PMC7203271
- DOI: 10.1007/s40122-019-00143-6
A Narrative Pharmacological Review of Buprenorphine: A Unique Opioid for the Treatment of Chronic Pain
Abstract
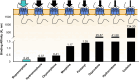
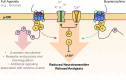
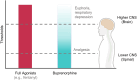
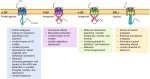
Buprenorphine is a Schedule III opioid analgesic with unique pharmacodynamic and pharmacokinetic properties that may be preferable to those of Schedule II full μ-opioid receptor agonists. The structure of buprenorphine allows for multimechanistic interactions with opioid receptors μ, δ, κ, and opioid receptor-like 1. Buprenorphine is considered a partial agonist with very high binding affinity for the μ-opioid receptor, an antagonist with high binding affinity for the δ- and κ-opioid receptors, and an agonist with low binding affinity for the opioid receptor-like 1 receptor. Partial agonism at the μ-opioid receptor does not provide partial analgesia, but rather analgesia equivalent to that of full μ-opioid receptor agonists. In addition, unlike full μ-opioid receptor agonists, buprenorphine may have a unique role in mediating analgesic signaling at spinal opioid receptors while having less of an effect on brain receptors, potentially limiting classic opioid-related adverse events such as euphoria, addiction, or respiratory depression. The pharmacokinetic properties of buprenorphine are also advantageous in a clinical setting, where metabolic and excretory pathways allow for use in patients requiring concomitant medications, the elderly, and those with renal or hepatic impairment. The unique pharmacodynamic and pharmacokinetic properties of buprenorphine translate to an effective analgesic with a potentially favorable safety profile compared with that of full μ-opioid receptor agonists for the treatment of chronic pain.
Keywords: Buprenorphine; Chronic pain; Opioid receptor; Pharmacodynamics; Pharmacokinetics; Pharmacology.
Plain language summary
The unique pharmacodynamic and pharmacokinetic properties of the Schedule III opioid buprenorphine contribute to its effective pain relief and a potentially favorable safety profile for chronic pain management.
Conflict of interest statement
In the past year, Jeffrey Gudin has served as a consultant for Averitas, Mallinckrodt, Nektar, and Quest Diagnostics; as an advisory board member for AcelRx Pharmaceuticals and GlaxoSmithKline; and as a consultant and part of a speakers' bureau for BioDelivery Sciences International, DSI, Salix Pharmaceuticals, and Scilex Pharmaceuticals. Jeffrey Fudin has served as an advisory board member for AcelRx Pharmaceuticals, GlaxoSmithKline, Quest Diagnostics, Scilex Pharmaceuticals, and Salix Pharmaceuticals; as a speaker for Acutis Diagnostics; as part of a speakers' bureau for AstraZeneca; as a consultant for BioDelivery Sciences International and Firstox Laboratories; and as part of an advisory board and speakers' bureau for Daiichi Sankyo.
Figures
References
-
- US Health and Human Services. HHS acting secretary declares public health emergency to address national opioid crisis. Available at: https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-.... Accessed Jan 24, 2019.
-
- National Institute on Drug Abuse. Opioid overdose crisis. Updated January 2019. Available at: https://www.drugabuse.gov/drugs-abuse/opioids/opioid-overdose-crisis. Accessed March 20, 2019.
-
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2017 national survey on drug use and health (HHS Publication No. SMA 18-5068, NSDUH Series H-53). 2018. Available at: https://www.samhsa.gov/data/report/2017-nsduh-annual-national-report. Accessed March 20, 2019.
-
- US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. 2019. Available at: https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf. Accessed July 1, 2019.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

