Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer
- PMID: 31994181
- PMCID: PMC6987034
- DOI: 10.1002/14651858.CD012988.pub2
Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer
Abstract
Background: Survival for stage I to III, hormone receptor-positive, breast cancer has substantially improved over time due to advances in screening, surgery and adjuvant therapy. However many adjuvant therapies have significant treatment-related toxicities, which worsen quality of life for breast cancer survivors. Postmenopausal women with hormone receptor-positive breast cancer are now prescribed aromatase inhibitors (AI) as standard, with longer durations of therapy, up to 10 years, being considered for certain women. AI treatment is associated with a high incidence of AI-induced musculoskeletal symptoms (AIMSS), often described as symmetrical pain and soreness in the joints, musculoskeletal pain and joint stiffness. AIMSS reduces compliance with AI therapy in up to one half of women undergoing adjuvant AI therapy, potentially compromising breast cancer outcomes. Exercise has been investigated for the prevention and treatment of AIMSS but the effect of this intervention remains unclear.
Objectives: To assess the effects of exercise therapies on the prevention or management of aromatase inhibitor-induced musculoskeletal symptoms (AIMSS) in women with stage I to III hormone receptor-positive breast cancer.
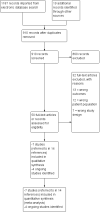
Search methods: We searched Cochrane Breast Cancer's Specialised Register, CENTRAL, MEDLINE, Embase and CINAHL databases up to 13 December 2018. We also searched two conference proceedings portals and two clinical trials registries for ongoing studies or unpublished trials, or both, in August 2019. We also reviewed reference lists of the included studies.
Selection criteria: We included randomised controlled trials that compared exercise versus a comparator arm. We did not impose any restriction on the comparator arm, which could include an alternative type of exercise, no exercise or a waiting list control. Both published and non-peer-reviewed studies were eligible.
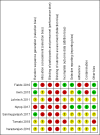
Data collection and analysis: Two review authors independently extracted data, assessed risk of bias and certainty of the evidence using the GRADE approach. The outcomes investigated were pain, joint stiffness, grip strength, health-related quality of life, cancer-specific quality of life, adherence to AI therapy, adverse events, incidence of AIMSS, breast cancer-specific survival and overall survival. For continuous outcomes that were assessed with the same instrument, we used the mean difference (MD); for those outcomes that used different instruments, we used the standardised mean difference (SMD) for the analysis. For dichotomous outcomes, we reported outcomes as an odds ratio (OR).
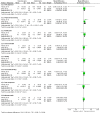
Main results: We included seven studies with 400 randomised participants; one study assessed exercise for preventing AIMSS and six studies assessed treating AIMSS. For preventing AIMSS, the single study reported no difference in pain scores, grip strength or compliance to taking AI medication between groups. Data values were not provided in the study and no other outcomes were reported. For managing AIMSS, we found that the evidence for the effect of exercise therapies on overall change in worst pain scores was very uncertain (SMD -0.23, 95% confidence interval (CI) -0.78 to 0.32; 4 studies, 284 women; very low-certainty evidence). The evidence suggested that exercise therapies result in little to no difference in overall change in stiffness scores (Western Ontario McMasters Universities Osteoarthritis Index (WOMAC) stiffness score MD -0.76, 95% CI -1.67 to 0.15 and Visual Analogues Scale (VAS) stiffness score MD -0.42, 95% CI -2.10 to 1.26; 1 study, 53 women; low-certainty evidence). The evidence was very uncertain for the outcomes of overall change in grip strength (MD 0.30, 95% CI -0.55 to 1.15; 1 study, 83 women; very low-certainty evidence); overall change in health-related quality of life (subscales of SF-36 tool ranged from least benefit of MD 1.88, 95% CI -2.69 to 6.45 to most benefit of MD 9.70, 95% CI 1.67 to 17.73; 2 studies, 123 women, very low-certainty evidence); overall change in cancer-specific quality of life (MD 4.58, 95% CI -0.61 to 9.78; 2 studies, 136 women; very low-certainty evidence); and adherence to aromatase inhibitors (OR 2.43, 95% CI 0.41 to 14.63; 2 studies, 224 women; very low-certainty evidence). There were no adverse events identified across four studies in either arm (0 events reported; 4 studies; 331 participants; low-certainty evidence). There were no data reported on incidence of AIMSS, breast cancer-specific survival or overall survival.
Authors' conclusions: Given the wide-ranging benefits of exercise for people affected by cancer, it was surprising that this review provided no clear evidence of benefit for exercise therapies in women with early breast cancer with AIMSS. This review only yielded seven eligible studies with 400 participants, which is likely to have underpowered the findings. The meta-analysis was challenging due to the considerable heterogeneity amongst the trials, with a wide range of exercise regimens and follow-up periods. Despite these inconclusive findings, exercise needs to be part of routine care for women with breast cancer due to its wide-ranging benefits. Future research in this area would be enhanced with further understanding of the mechanism of AIMSS, a single clear definition of the condition, and phase III randomised controlled trials that are adequately powered to test targeted exercise interventions on the key clinical outcomes in this condition.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
KER: received sponsorship from Roche, Amgen & BMS for accommodation, travel and registration to educational meetings. This financial support is unrelated to the topic under review. KR: none to declare DV: none to declare SF: travel/accommodation by Eisai. This financial support is unrelated to the topic under review. NW: consultancy fees from Roche, Novartis; grants from Medivation; expert panel review for Roche and Pfizer; travel/accommodation/meeting expenses for Roche, Novartis; stock in CSL. While both Pfizer and Novartis do market aromatase inhibitors (and these medicines are now off patent), this review focuses on managing side effects rather than assessing their efficacy. Relationship with Roche is not relevant to a review of managing aromatase inhibitor side effects.
Figures

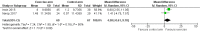








Update of
- doi: 10.1002/14651858.CD012988
Similar articles
-
Psychosocial interventions for preventing and treating depression in dialysis patients.Cochrane Database Syst Rev. 2019 Dec 2;12(12):CD004542. doi: 10.1002/14651858.CD004542.pub3. Cochrane Database Syst Rev. 2019. PMID: 31789430 Free PMC article.
-
Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer.Cochrane Database Syst Rev. 2020 Mar 6;3(3):CD013538. doi: 10.1002/14651858.CD013538. Cochrane Database Syst Rev. 2020. PMID: 32141074 Free PMC article.
-
Acupuncture and related interventions for the treatment of symptoms associated with carpal tunnel syndrome.Cochrane Database Syst Rev. 2018 Dec 2;12(12):CD011215. doi: 10.1002/14651858.CD011215.pub2. Cochrane Database Syst Rev. 2018. PMID: 30521680 Free PMC article.
-
Exercise for cancer cachexia in adults.Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD010804. doi: 10.1002/14651858.CD010804.pub3. Cochrane Database Syst Rev. 2021. PMID: 33735441 Free PMC article.
-
Mindfulness-based stress reduction for women diagnosed with breast cancer.Cochrane Database Syst Rev. 2019 Mar 27;3(3):CD011518. doi: 10.1002/14651858.CD011518.pub2. Cochrane Database Syst Rev. 2019. PMID: 30916356 Free PMC article.
Cited by
-
Rheumatoid Arthritis-Like Symptoms After Taking Relugolix, With Primary Exacerbation After Discontinuation of the Drug.Cureus. 2024 Feb 4;16(2):e53584. doi: 10.7759/cureus.53584. eCollection 2024 Feb. Cureus. 2024. PMID: 38318276 Free PMC article.
-
Endocrine therapy with accelerated Partial breast irradiatiOn or exclusive ultra-accelerated Partial breast irradiation for women aged ≥ 60 years with Early-stage breast cancer (EPOPE): The rationale for a GEC-ESTRO randomized phase III-controlled trial.Clin Transl Radiat Oncol. 2021 Apr 22;29:1-8. doi: 10.1016/j.ctro.2021.04.005. eCollection 2021 Jul. Clin Transl Radiat Oncol. 2021. PMID: 33997321 Free PMC article.
-
Person-centred support programme (RESPECT intervention) for women with breast cancer treated with endocrine therapy: a feasibility study.BMJ Open. 2022 Oct 5;12(10):e060946. doi: 10.1136/bmjopen-2022-060946. BMJ Open. 2022. PMID: 36198470 Free PMC article. Clinical Trial.
-
Aromatase inhibitor-associated musculoskeletal pain: An overview of pathophysiology and treatment modalities.SAGE Open Med. 2022 Mar 19;10:20503121221078722. doi: 10.1177/20503121221078722. eCollection 2022. SAGE Open Med. 2022. PMID: 35321462 Free PMC article. Review.
-
The effectiveness of exercise on the symptoms in breast cancer patients undergoing adjuvant treatment: an umbrella review of systematic reviews and meta-analyses.Front Oncol. 2023 Sep 20;13:1222947. doi: 10.3389/fonc.2023.1222947. eCollection 2023. Front Oncol. 2023. PMID: 37799468 Free PMC article. Review.
References
References to studies included in this review
Fields 2016 {published data only}
-
- Fields J, Richardson A, Hopkinson J, Fenlon D. Nordic walking as an exercise intervention to reduce pain in women with aromatase inhibitor‐ associated arthralgia: a feasibility study. Journal of Pain and Symptom Management 2016;52(4):548‐59. - PubMed
Irwin 2015 {published and unpublished data}
-
- Crespo‐Bosque M, Brown C, Cartmel B, Harrigan M, Irwin M, Neogi T. Pain and sensitization in women with aromatase inhibitor‐associated arthralgias. Arthritis and Rheumatology 2016;68:2524‐6.
-
- Irwin M. Exercise in the management of arthralgia. Asia‐Pacific Journal of Clinical Oncology 2016;12:69.
Lohrisch 2011 {published data only}
-
- Lohrisch CA, McKenzie D, Truong P, Jesperson D, Gelmon KA, Premji S, et al. A randomized trial of exercise versus control for musculoskeletal symptoms from adjuvant anastrozole (A) for postmenopausal early breast cancer (PEBC). Journal of Clinical Oncology 2011;29(15 Suppl):636.
Nyrop 2017 {published data only (unpublished sought but not used)}
Sanmugarajah 2017 {published data only}
-
- Sanmugarajah J, Allan S, Bagchi R, Laakso EL. Can a supervised exercise program compared to usual care prevent aromatase inhibitor‐induced musculoskeletal pain in women with breast cancer?. Cancer Research 2017;77(4 Suppl):P5‐12‐02.
Tamaki 2018 {published data only (unpublished sought but not used)}
-
- Tamaki K, Takaesu M, Nagamine S, Terukina S, Kamada Y, Uehara K, et al. Final results of the randomized trial of exercise intervention vs. usual care for breast cancer patients with aromatase inhibitor to prevent and improve the aromatase inhibitor induced arthralgia. Cancer Research 2018;78(4 Suppl):P6‐11‐01.
Varadarajan 2016 {published data only}
-
- Varadarajan R, Helm E, Arnold C, Huelsenbeck‐Dill L, Ingraham‐Lopresto B, Sonaad S, et al. Abstract P5‐12‐04: directed exercise intervention in breast cancer patients with arthralgias receiving aromatase inhibitors: a randomized pilot study. Cancer Research 2016;76(4 Supplement):P5‐12‐04‐P5‐12‐04.
References to studies excluded from this review
Bower 2012 {published data only}
Brown 2015 {published data only}
Cantarero‐Villanueva 2013a {published data only}
-
- Cantarero‐Villanueva I, Fernandez‐Lao C, Caro‐Moran E, Morillas‐Ruiz J, Galiano‐Castillo N, Diaz‐Rodriguez L, et al. Aquatic exercise in a chest‐high pool for hormone therapy‐induced arthralgia in breast cancer survivors: a pragmatic controlled trial. Clinical Rehabilitation 2013;27(2):123‐32. - PubMed
Cantarero‐Villanueva 2013b {published data only}
-
- Cantarero‐Villanueva I, Fernandez‐Lao C, Cuesta‐Vargas A I, Moral‐Avila R, Fernandez‐de‐Las‐Penas C, Arroyo‐Morales M. The effectiveness of a deep water aquatic exercise program in cancer‐related fatigue in breast cancer survivors: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2013;94(2):221‐30. - PubMed
DeNysschen 2014 {published data only}
-
- DeNysschen CA, Burton H, Ademuyiwa F, Levine E, Tetewsky S, O'Connor T. Exercise intervention in breast cancer patients with aromatase inhibitor‐associated arthralgia: a pilot study. European Journal of Cancer Care 2014;23(4):493‐501. - PubMed
Desbiens 2017 {published data only}
-
- Desbiens C, Filion M, Brien MC, Hogue JC, Laflamme C, Lemieux J. Impact of physical activity in group versus individual physical activity on fatigue in patients with breast cancer: a pilot study. Breast 2017;35:8‐13. - PubMed
Djuric 2011 {published data only}
Galantino 2013 {published data only}
-
- Galantino M, Cardena G, Piela N, Callens M, Mao JJ. Impact of tai chi on quality of life in breast cancer survivors with aromatase inhibitor associated arthralgia. Rehabilitation Oncology 2013;31(1):50‐1. - PubMed
Galiano‐Castillo 2017 {published data only}
-
- Galiano‐Castillo N, Arroyo‐Morales M, Lozano‐Lozano M, Fernandez‐Lao C, Martin‐Martin L, Del‐Moral‐Avila R, et al. Effect of an internet‐based telehealth system on functional capacity and cognition in breast cancer survivors: a secondary analysis of a randomized controlled trial. Supportive Care in Cancer 2017;25(11):3551‐9. - PubMed
Goodwin 2014 {published data only}
-
- Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. Randomized trial of a telephone‐based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. Journal of Clinical Oncology 2014;32(21):2231‐9. - PubMed
Harrigan 2016 {published data only}
-
- Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized trial comparing telephone versus in‐person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the Lifestyle, Exercise, and Nutrition (LEAN) Study. Journal of Clinical Oncology 2016;34(7):669‐76. - PMC - PubMed
Kiecolt‐Glaser 2014 {published data only}
Knobf 2017 {published data only}
Lash 2011 {published data only}
-
- Lash BW, Katz J, Gilman P. Feasibility of a defined exercise program for aromatase inhibitor–related arthralgia (AIRA) in breast cancer survivors. Journal of Clinical Oncology 2011;29(15 Suppl):e19725.
Ligibel 2008 {published data only}
-
- Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. Journal of Clinical Oncology 2008;26(6):907‐12. - PubMed
Ligibel 2011 {published data only}
-
- Ligibel JA, Segal R, Pond G, Dion MJ, Pritchard KI, Levine M, et al. Impact of the lifestyle intervention study in adjuvant treatment of early breast cancer (LISA) weight loss intervention upon physical activity. Cancer Research 2011;71(24 Suppl 3):P4‐12‐05.
Nikander 2012 {published data only}
-
- Nikander R, Sievanen H, Ojala K, Kellokumpu‐Lehtinen PL, Palva T, Blomqvist C, et al. Effect of exercise on bone structural traits, physical performance and body composition in breast cancer patients‐‐a 12‐month RCT. Journal of Musculoskeletal & Neuronal Interactions 2012;12(3):127‐35. - PubMed
Nyrop 2016 {published data only}
-
- Nyrop KA, Callahan LF, Rini C, Altpeter M, Hackney B, DePue A, et al. Aromatase inhibitor associated arthralgia: the importance of oncology provider‐patient communication about side effects and potential management through physical activity. Supportive Care in Cancer 2016;24(6):2643‐50. - PMC - PubMed
Pakiz 2016 {published data only}
Paulo 2019 {published data only}
Payne 2008 {published data only}
-
- Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncology Nursing Forum 2008;35(4):635‐42. - PubMed
Penttinen 2009 {published data only}
-
- Penttinen H, Nikander R, Blomqvist C, Luoto R, Saarto T. Recruitment of breast cancer survivors into a 12‐month supervised exercise intervention is feasible. Contemporary Clinical Trials 2009;30(5):457‐63. - PubMed
Peppone 2012 {published data only}
-
- Peppone LJ, Mohile SG, Janelsins MC, Sprod L, Gewandter JS, Heckler CE, et al. YOCAS yoga for musculoskeletal symptoms in breast cancer patients receiving aromatase inhibitors: A URCC CCOP randomized, controlled clinical trial. Journal of Clinical Oncology 2012;30(15 Suppl):9028.
Peppone 2015 {published data only}
Pruthi 2012 {published data only}
-
- Pruthi S, Stan DL, Jenkins SM, Huebner M, Borg BA, Thomley BS, et al. A randomized controlled pilot study assessing feasibility and impact of yoga practice on quality of life, mood, and perceived stress in women with newly diagnosed breast cancer. Global Advances in Health and Medicine 2012;1(5):30‐5. - PMC - PubMed
Reeves 2017 {published data only}
-
- Reeves M, Winkler E, McCarthy N, Lawler S, Terranova C, Hayes S, et al. The Living Well after Breast Cancer pilot trial: a weight loss intervention for women following treatment for breast cancer. Asia‐Pacific Journal of Clinical Oncology 2017;13(3):125‐36. - PubMed
Rogers 2009 {published data only}
-
- Rogers LQ, Hopkins‐Price P, Vicari S, Markwell S, Pamenter R, Courneya KS, et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: persistent and delayed effects. Cancer Epidemiology, Biomarkers & Prevention 2009;18(5):1410‐8. - PubMed
Rogers 2009a {published data only}
-
- Rogers LQ, Hopkins‐Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, et al. A randomized trial to increase physical activity in breast cancer survivors. Medicine and Science in Sports and Exercise 2009;41(4):935‐46. - PubMed
Segal 2011 {published data only}
-
- Segal R, Pond GR, Vallis M, Dion M, Pritchard KI, Ligibel JA, et al. Randomized trial of a lifestyle intervention for women with early‐stage breast cancer (BC) receiving adjuvant hormone therapy: initial results. Journal of Clinical Oncology 2011;29(15 Suppl 1):512.
Winkels 2017 {published data only}
-
- Winkels RM, Sturgeon KM, Kallan MJ, Dean LT, Zhang Z, Evangelisti M, et al. The women in steady exercise research (WISER) survivor trial: the innovative transdisciplinary design of a randomized controlled trial of exercise and weight‐loss interventions among breast cancer survivors with lymphedema. Contempory Clinical Trials 2017;61:63‐72. - PMC - PubMed
Winters‐Stone 2012 {published data only}
References to ongoing studies
NCT03162133 {published data only}
-
- NCT03162133. A randomized controlled trial evaluating the impact of Baduanjin exercise intervention on quality of life in breast cancer survivors receiving aromatase inhibitor therapy. clinicaltrials.gov/ct2/show/NCT03162133 (first received 22 May 2017).
NCT03786198 {published data only}
-
- NCT03786198. Activity program during aromatase inhibitor therapy. clinicaltrials.gov/ct2/show/NCT03786198 (first received 24 December 2018).
NCT03953157 {published data only}
-
- NCT03953157. Dietary and exercise interventions in reducing side effects in patients with stage I‐IIIa breast cancer receiving aromatase inhibitors. clinicaltrials.gov/ct2/show/NCT03953157 (first received 16 May 2019).
NCT03956875 {published data only}
-
- NCT03956875. Yoga for aromatase inhibitor‐induced knee pain relief in breast cancer patients. clinicaltrials.gov/ct2/show/NCT03956875 (first received 21 May 2019).
Additional references
Aydiner 2013
-
- Aydiner A. Meta‐analysis of breast cancer outcome and toxicity in adjuvant trials of aromatase inhibitors in postmenopausal women. Breast 2013;22(2):121‐9. - PubMed
Baglia 2019
Beckwee 2017
-
- Beckwee D, Leysen L, Meuwis K, Adriaenssens N. Prevalence of aromatase inhibitor‐induced arthralgia in breast cancer: a systematic review and meta‐analysis. Supportive Care in Cancer 2017;25(5):1673‐86. - PubMed
Brier 2017
Burstein 2007
-
- Burstein HJ. Aromatase inhibitor‐associated arthralgia syndrome. Breast 2007;16(3):223‐34. - PubMed
Burstein 2019
-
- Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, et al. Adjuvant endocrine therapy for women with hormone receptor‐positive breast cancer: ASCO Clinical Practice Guideline Focused Update. Journal of Clinical Oncology 2019;37(5):423‐38. - PubMed
Campbell 2019
Caspersen 1985
Castel 2015
Chim 2013
Cormie 2017
-
- Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment‐related adverse effects. Epidemiologic Reviews 2017;39(1):71‐92. - PubMed
COSA 2018
-
- Clinical Oncology Society of Australia. COSA Position Statement on Exercise in Cancer Care. Sydney: Clinical Oncology Society of Australia, 2018.
Cramp 2012
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dennett 2016
-
- Dennett AM, Peiris CL, Shields N, Prendergast LA, Taylor NF. Moderate‐intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta‐regression. Journal of Physiotherapy 2016;62(2):68‐82. - PubMed
Deshpande 2011
EBCTCG 2015
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient‐level meta‐analysis of the randomised trials. Lancet 2015;386(10001):1341‐52. - PubMed
Eton 2004
-
- Eton DT, Cella D, Yost KJ, Yount SE, Peterman AH, Neuberg DS, et al. A combination of distribution‐and anchor‐based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. Journal of Clinical Epidemiology 2004;57(9):898‐910. - PubMed
European Society for Medical Oncology 2014
-
- European Society for Medical Oncology. ESMO Handbook on Rehabilitation Issues During Cancer Treatment and Follow‐Up. Lugano, Switzerland: European Society for Medical Oncology, 2014.
Excel [Computer program]
-
- Microsoft. Excel. Microsoft, 2016.
Ferlay 2012
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. International Agency for Research on Cancer. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. globocan.iarc.fr. Lyon, France: International Agency for Research on Cancer, (accessed prior to 14 March 2018). [http://globocan.iarc.fr,]
Fields 2015
-
- Fields J. A feasibility study of a Nordic walking intervention for women experiencing aromatase inhibitor associated arthralgia [Doctoral Thesis]. Southampton, U.K.: University of Southampton, 2015.
Francis 2015
Francis 2018
Fransen 2014
Furmaniak 2016
Gerritsen 2016
-
- Gerritsen JK, Vincent AJ. Exercise improves quality of life in patients with cancer: a systematic review and meta‐analysis of randomised controlled trials. British Journal of Sports Medicine 2016;50(13):796‐803. - PubMed
GRADEpro GDT [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed October 2017. Hamilton (ON): GRADE Working Group, McMaster University.
Hadji 2014
-
- Hadji P, Jackisch C, Bolten W, Blettner M, Hindenburg HJ, Klein P, et al. COMPliance and Arthralgia in Clinical Therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Annals of Oncology 2014;25(2):372‐7. - PubMed
Hawe 2004a
Hawe 2004b
Henry 2012
Hershman 2011
Hershman 2015
-
- Hershman DL, Loprinzi C, Schneider BP. Symptoms: aromatase inhibitor induced arthralgias. In: Ganz PA editor(s). Improving Outcomes for Breast Cancer Survivors: Perspectives on Research Challenges and Opportunities. Cham: Springer International Publishing, 2015:89‐100. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.. Available from www.handbook.cochrane.org..
Higgins 2011b
-
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.. Available from www.handbook.cochrane.org..
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Howlader 2019
-
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. Table 1.11 Median age of cancer patients at diagnosis (2012‐2016). SEER Cancer Statistics Review, (1975‐2016). Bethesda, MD: National Cancer Institute., 2019. [ http://seer.cancer.gov/csr/1975_2016/ Accessed on August 6, 2019]
IntHout 2014
Irwin 2009
-
- Irwin ML. Physical activity interventions for cancer survivors. British Journal of Sports Medicine 2009;43(1):32‐8. - PubMed
Kadakia 2016
Kemp‐Casey 2017
-
- Kemp‐Casey A, Roughead EE, Saunders C, Boyle F, Bulsara M, Preen DB. Switching between endocrine therapies for primary breast cancer: frequency and timing in Australian clinical practice. Asia‐Pacific Journal of Clinical Oncology 2017;13(2):e161‐70. - PubMed
Kilari 2016
Kim 2018
-
- Kim TH, Kang JW, Lee TH. Therapeutic options for aromatase inhibitor‐associated arthralgia in breast cancer survivors: a systematic review of systematic reviews, evidence mapping, and network meta‐analysis. Maturitas 2018;118:29‐37. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.. Available from www.handbook.cochrane.org.
Lintermans 2013
-
- Lintermans A, Laenen A, Calster B, Hoydonck M, Pans S, Verhaeghe J, et al. Prospective study to assess fluid accumulation and tenosynovial changes in the aromatase inhibitor‐induced musculoskeletal syndrome: 2‐year follow‐up data. Annals of Oncology 2013;24(2):350‐5. - PubMed
Mao 2009
Medical Research Council 2000
-
- Medical Research Council. A Framework for development for the development and evaluation of randomised controlled trials for complex interventions to improve health. London: Medical Research Council, 2000.
Merriam‐Webster
-
- Merriam‐Webster Dictionary. Definition of therapy. www.merriam‐webster.com (accessed 8 March 2018). [www.merriam‐webster.com/]
Mishra 2012
Moher 2009
Nadji 2005
-
- Nadji M, Gomez‐Fernandez C, Ganjei‐Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. American Journal of Clinical Pathology 2005;123(1):21‐7. - PubMed
Nahm 2018
-
- Nahm N, Mee S, Marx G. Efficacy of management strategies for aromatase inhibitor‐induced arthralgia in breast cancer patients: a systematic review. Asia‐Pacific Journal of Clinical Oncology 2018;14(6):374‐82. - PubMed
Nipp 2017
-
- Nipp R, Temel J. The patient knows best: incorporating patient‐reported outcomes into routine clinical care. Journal of the National Cancer Institute 2017;109(9):djx044. - PubMed
Niravath 2013
-
- Niravath P. Aromatase inhibitor‐induced arthralgia: a review. Annals of Oncology 2013;24(6):1443‐9. - PubMed
Osteras 2017
Partridge 2008
-
- Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis‐Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early‐stage breast cancer. Journal of Clinical Oncology 2008;26(4):556‐62. - PubMed
Patsou 2017
Peddle‐McIntyre 2019
Presant 2007
-
- Presant CA, Bosserman L, Young T, Vakil M, Horns R, Upadhyaya G, et al. Aromatase inhibitor‐associated arthralgia and/or bone pain: frequency and characterization in non‐clinical trial patients. Clinical Breast Cancer 2007;7(10):775‐8. - PubMed
Rand Health Care
-
- Rand Health Care. 36‐Item Short Form Survey (SF‐36). www.rand.org/health‐care/surveys_tools/mos/36‐item‐short‐form.html. [www.rand.org/health‐care/surveys_tools/mos/36‐item‐short‐form.html]
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2017
-
- Roberts K, Rickett K, Greer R, Woodward N. Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early breast cancer: a systematic review and meta‐analysis. Critical Reviews in Oncology/Hematology 2017;111:66‐80. - PubMed
Rock 2012
-
- Rock CL, Doyle C, Demark‐Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA: a Cancer Journal for Clinicians 2012;62(4):243‐74. - PubMed
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
SIGN
-
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html#random2011.
Smaradottir 2017
-
- Smaradottir A, Smith AL, Borgert AJ, Oettel KR. Are we on the same page? Patient and provider perceptions about exercise in cancer care: a focus group study. Journal of the National Comprehensive Cancer Network 2017;15(5):588‐94. - PubMed
Szlezak 2016
-
- Szlezak AM, Szlezak SL, Keane J, Tajouri L, Minahan C. Establishing a dose‐response relationship between acute resistance‐exercise and the immune system: protocol for a systematic review. Immunology Letters 2016;180:54‐65. - PubMed
Tajaesu 2017
-
- Tajaesu M, Tamaki K, Nagamine S, Kamada Y, Uehara K, Arakaki M, et al. Randomized trial of exercise intervention vs. usual care for breast cancer patients with aromatase inhibitor to prevent and improve the aromatase inhibitor induced arthralgia. Cancer Research 2017;77(4 Suppl):P5‐12‐01.
Turner 2018
Ward 2014
Yang 2017
-
- Yang GS, Kim HJ, Griffith KA, Zhu S, Dorsey SG, Renn CL. Interventions for the treatment of aromatase inhibitor‐associated arthralgia in breast cancer survivors: a systematic review and meta‐analysis. Cancer Nursing 2017;40(4):E26‐e41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

