SIRT6 is a DNA double-strand break sensor
- PMID: 31995034
- PMCID: PMC7051178
- DOI: 10.7554/eLife.51636
SIRT6 is a DNA double-strand break sensor
Abstract
DNA double-strand breaks (DSB) are the most deleterious type of DNA damage. In this work, we show that SIRT6 directly recognizes DNA damage through a tunnel-like structure that has high affinity for DSB. SIRT6 relocates to sites of damage independently of signaling and known sensors. It activates downstream signaling for DSB repair by triggering ATM recruitment, H2AX phosphorylation and the recruitment of proteins of the homologous recombination and non-homologous end joining pathways. Our findings indicate that SIRT6 plays a previously uncharacterized role as a DNA damage sensor, a critical factor in initiating the DNA damage response (DDR). Moreover, other Sirtuins share some DSB-binding capacity and DDR activation. SIRT6 activates the DDR before the repair pathway is chosen, and prevents genomic instability. Our findings place SIRT6 as a sensor of DSB, and pave the road to dissecting the contributions of distinct DSB sensors in downstream signaling.
Keywords: DNA damage; DNA repair; DSB; SIRT6; cell biology; human; sensor.
Plain language summary
DNA is a double-stranded molecule in which the two strands run in opposite directions, like the lanes on a two-lane road. Also like a road, DNA can be damaged by use and adverse conditions. Double-strand breaks – where both strands of DNA snap at once – are the most dangerous type of DNA damage, so cells have systems in place to rapidly detect and repair this kind of damage. There are three confirmed sensors for double-strand break in human cells. A fourth protein, known as SIRT6, arrives within five seconds of DNA damage, and was known to make the DNA more accessible so that it can be repaired. However, it was unclear whether SIRT6 could detect the double-strand break itself, or whether it was recruited to the damage by another double-strand break sensor. To address this issue, Onn et al. blocked the three other sensors in human cells and watched the response to DNA damage. Even when all the other sensors were inactive, SIRT6 still arrived at damaged DNA and activated the DNA damage response. To find out how SIRT6 sensed DNA damage, Onn et al. examined how purified SIRT6 interacts with different kinds of DNA. This revealed that SIRT6 sticks to broken DNA ends, especially if the end of one strand slightly overhangs the other – a common feature of double-strand breaks. A closer look at the structure of the SIRT6 protein revealed that it contains a narrow tube, which fits over the end of one broken DNA strand. When both strands break at once, two SIRT6 molecules cap the broken ends, joining together to form a pair. This pair not only protects the open ends of the DNA from further damage, it also sends signals to initiating repairs. In this way, SIRT6 could be thought of acting like a paramedic who arrives first on the scene of an accident and works to treat the injured while waiting for more specialized help to arrive. Understanding the SIRT6 sensor could improve knowledge about how cells repair their DNA. SIRT6 arrives before the cell chooses how to fix its broken DNA, so studying it further could reveal how that critical decision happens. This is important for medical research because DNA damage builds up in age-related diseases like cancer and neurodegeneration. In the long term, these findings can help us develop new treatments that target different types of DNA damage sensors.
© 2020, Onn et al.
Conflict of interest statement
LO, MP, SI, GC, DS, SK, IS, ZS, ME, FE, BA, DT No competing interests declared
Figures
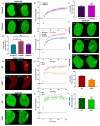
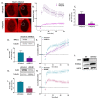
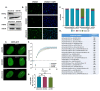

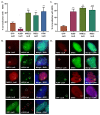

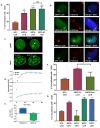


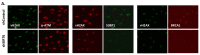
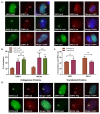




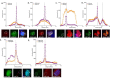

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

