Prognostic Models Derived in PARADIGM-HF and Validated in ATMOSPHERE and the Swedish Heart Failure Registry to Predict Mortality and Morbidity in Chronic Heart Failure
- PMID: 31995119
- PMCID: PMC6990745
- DOI: 10.1001/jamacardio.2019.5850
Prognostic Models Derived in PARADIGM-HF and Validated in ATMOSPHERE and the Swedish Heart Failure Registry to Predict Mortality and Morbidity in Chronic Heart Failure
Erratum in
-
Error in Abstract.JAMA Cardiol. 2020 Apr 1;5(4):488. doi: 10.1001/jamacardio.2020.0451. JAMA Cardiol. 2020. PMID: 32159732 Free PMC article. No abstract available.
Abstract
Importance: Accurate prediction of risk of death or hospitalizations in patients with heart failure (HF) may allow physicians to explore how more accurate decisions regarding appropriateness and timing of disease-modifying treatments, advanced therapies, or the need for end-of-life care can be made.
Objective: To develop and validate a prognostic model for patients with HF.
Design, setting, and participants: Multivariable analyses were performed in a stepwise fashion. Harrell C statistic was used to assess the discriminative ability. The derivation cohort was Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM-HF) participants. The models were validated using the Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure Trial (ATMOSPHERE) study and in the Swedish Heart Failure Registry (SwedeHF). A total of 8399 participants enrolled in PARADIGM-HF. Data were analyzed between June 2016 and June 2018.
Main outcomes and measures: Cardiovascular death, all-cause mortality, and the composite of cardiovascular death or HF hospitalization at both 1 and 2 years.
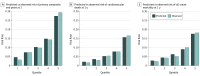
Results: Complete baseline clinical data were available for 8011 patients in PARADIGM-HF. The mean (SD) age of participants was 64 (11.4) years, 78.2% were men (n = 6567 of 8399), and 70.6% were New York Heart Association class II (n = 5919 of 8399). During a mean follow-up of 27 months, 1546 patients died, and 2031 had a cardiovascular death or HF hospitalization. The common variables were: male sex, race/ethnicity (black or Asian), region (Central Europe or Latin America), HF duration of more than 5 years, New York Heart Association class III/ IV, left ventricular ejection fraction, diabetes mellitus, β-blocker use at baseline, and allocation to sacubitril/valsartan. Ranked by χ2, N-terminal pro brain natriuretic peptide was the single most powerful independent predictor of each outcome. The C statistic at 1 and 2 years was 0.74 (95% CI, 0.71-0.76) and 0.71 (95% CI, 0.70-0.73) for the primary composite end point, 0.73 (95% CI, 0.71-0.75) and 0.71 (95% CI, 0.69-0.73) for cardiovascular death, and 0.71 (95% CI, 0.69-0.74) and 0.70 (95% CI, 0.67-0.74) for all-cause death, respectively. When validated in ATMOSPHERE, the C statistic at 1 and 2 years was 0.71 (95% CI, 0.69-0.72) and 0.70 (95% CI, 0.68-0.71) for the primary composite end point, 0.71 (95% CI, 0.69-0.74) and 0.70 (95% CI, 0.69-0.72) for cardiovascular death, and 0.71 (95% CI, 0.69-0.74) and 0.70 (95% CI, 0.68-0.72) for all-cause death, respectively. An online calculator was created to allow calculation of an individual's risk (http://www.predict-hf.com).
Conclusions and relevance: Predictive models performed well and were developed and externally validated in large cohorts of geographically representative patients, comprehensively characterized with clinical and laboratory data including natriuretic peptides, who were receiving contemporary evidence-based treatment.
Conflict of interest statement
Figures

References
-
- Simpson J, Jhund PS, Silva Cardoso J, et al. ; PARADIGM-HF Investigators and Committees . Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS-HF risk scores: an analysis of mortality and morbidity in PARADIGM-HF. J Am Coll Cardiol. 2015;66(19):2059-2071. doi:10.1016/j.jacc.2015.08.878 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

