Short-term plasticity in glomerular inhibitory circuits shapes olfactory bulb output
- PMID: 31995427
- PMCID: PMC7099473
- DOI: 10.1152/jn.00628.2019
Short-term plasticity in glomerular inhibitory circuits shapes olfactory bulb output
Abstract
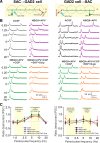
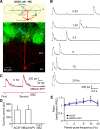
Short-term plasticity is a fundamental synaptic property thought to underlie memory and neural processing. The glomerular microcircuit comprises complex excitatory and inhibitory interactions and transmits olfactory nerve signals to the excitatory output neurons, mitral/tufted cells (M/TCs). The major glomerular inhibitory interneurons, short axon cells (SACs) and periglomerular cells (PGCs), both provide feedforward and feedback inhibition to M/TCs and have reciprocal inhibitory synapses between each other. Olfactory input is episodically driven by sniffing. We hypothesized that frequency-dependent short-term plasticity within these inhibitory circuits could influence signals sent to higher-order olfactory networks. To assess short-term plasticity in glomerular circuits and MC outputs, we virally delivered channelrhodopsin-2 (ChR2) in glutamic acid decarboxylase-65 promotor (GAD2-cre) or tyrosine hydroxylase promoter (TH-cre) mice and selectively activated one of these two populations while recording from cells of the other population or from MCs. Selective activation of TH-ChR2-expressing SACs inhibited all recorded GAD2-green fluorescent protein(GFP)-expressing presumptive PGC cells, and activation of GAD2-ChR2 cells inhibited TH-GFP-expressing SACs, indicating reciprocal inhibitory connections. SAC synaptic inhibition of GAD2-expressing cells was significantly facilitated at 5-10 Hz activation frequencies. In contrast, GAD2-ChR2 cell inhibition of TH-expressing cells was activation-frequency independent. Both SAC and PGC inhibition of MCs also exhibited short-term plasticity, pronounced in the 5-20 Hz range corresponding to investigative sniffing frequency ranges. In paired SAC and olfactory nerve electrical stimulations, the SAC to MC synapse was able to markedly suppress MC spiking. These data suggest that short-term plasticity across investigative sniffing ranges may differentially regulate intra- and interglomerular inhibitory circuits to dynamically shape glomerular output signals to downstream targets.NEW & NOTEWORTHY Short-term plasticity is a fundamental synaptic property that modulates synaptic strength based on preceding activity of the synapse. In rodent olfaction, sensory input arrives episodically driven by sniffing rates ranging from quiescent respiration (1-2 Hz) through to investigative sniffing (5-10 Hz). Here we show that glomerular inhibitory networks are exquisitely sensitive to input frequencies and exhibit plasticity proportional to investigative sniffing frequencies. This indicates that olfactory glomerular circuits are dynamically modulated by episodic sniffing input.
Keywords: STP; mitral cells; network; olfactory bulb; periglomerular cells; short axon cells.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Reciprocal Inhibitory Glomerular Circuits Contribute to Excitation-Inhibition Balance in the Mouse Olfactory Bulb.eNeuro. 2019 Jun 12;6(3):ENEURO.0048-19.2019. doi: 10.1523/ENEURO.0048-19.2019. Print 2019 May/Jun. eNeuro. 2019. PMID: 31147391 Free PMC article.
-
CCKergic Tufted Cells Differentially Drive Two Anatomically Segregated Inhibitory Circuits in the Mouse Olfactory Bulb.J Neurosci. 2020 Aug 5;40(32):6189-6206. doi: 10.1523/JNEUROSCI.0769-20.2020. Epub 2020 Jun 30. J Neurosci. 2020. PMID: 32605937 Free PMC article.
-
Muscarinic receptors modulate dendrodendritic inhibitory synapses to sculpt glomerular output.J Neurosci. 2015 Apr 8;35(14):5680-92. doi: 10.1523/JNEUROSCI.4953-14.2015. J Neurosci. 2015. PMID: 25855181 Free PMC article.
-
The circuits of the olfactory bulb. The exception as a rule.Anat Rec (Hoboken). 2013 Sep;296(9):1401-12. doi: 10.1002/ar.22732. Epub 2013 Jul 31. Anat Rec (Hoboken). 2013. PMID: 23907743 Review.
-
Dendritic processing within olfactory bulb circuits.Trends Neurosci. 2003 Sep;26(9):501-6. doi: 10.1016/S0166-2236(03)00228-5. Trends Neurosci. 2003. PMID: 12948662 Review.
Cited by
-
Co-transmitting interneurons in the mouse olfactory bulb regulate olfactory detection and discrimination.Cell Rep. 2023 Dec 26;42(12):113471. doi: 10.1016/j.celrep.2023.113471. Epub 2023 Nov 18. Cell Rep. 2023. PMID: 37980561 Free PMC article.
-
Molecular self-avoidance in synaptic neurexin complexes.Sci Adv. 2021 Dec 17;7(51):eabk1924. doi: 10.1126/sciadv.abk1924. Epub 2021 Dec 17. Sci Adv. 2021. PMID: 34919427 Free PMC article.
-
Sex steroid hormone synthesis, metabolism, and the effects on the mammalian olfactory system.Cell Tissue Res. 2023 Jan;391(1):19-42. doi: 10.1007/s00441-022-03707-9. Epub 2022 Nov 19. Cell Tissue Res. 2023. PMID: 36401093 Free PMC article. Review.
-
Sniff-synchronized, gradient-guided olfactory search by freely moving mice.Elife. 2021 May 4;10:e58523. doi: 10.7554/eLife.58523. Elife. 2021. PMID: 33942713 Free PMC article.
-
Cell type-specific and frequency-dependent centrifugal modulation in olfactory bulb output neurons in vivo.J Neurophysiol. 2024 Jun 1;131(6):1226-1239. doi: 10.1152/jn.00078.2024. Epub 2024 May 1. J Neurophysiol. 2024. PMID: 38691531 Free PMC article.
References
-
- Banerjee A, Marbach F, Anselmi F, Koh MS, Davis MB, Garcia da Silva P, Delevich K, Oyibo HK, Gupta P, Li B, Albeanu DF. An interglomerular circuit gates glomerular output and implements gain control in the mouse olfactory bulb. Neuron 87: 193–207, 2015. doi:10.1016/j.neuron.2015.06.019. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

