Cell cycle oscillators underlying orderly proteolysis of E2F8
- PMID: 31995441
- PMCID: PMC7185961
- DOI: 10.1091/mbc.E19-12-0725
Cell cycle oscillators underlying orderly proteolysis of E2F8
Abstract
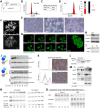
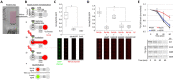
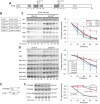
E2F8 is a transcriptional repressor that antagonizes E2F1 at the crossroads of the cell cycle, apoptosis, and cancer. Previously, we discovered that E2F8 is a direct target of the APC/C ubiquitin ligase. Nevertheless, it remains unknown how E2F8 is dynamically controlled throughout the entirety of the cell cycle. Here, using newly developed human cell-free systems that recapitulate distinct inter-mitotic and G1 phases and a continuous transition from prometaphase to G1, we reveal an interlocking dephosphorylation switch coordinating E2F8 degradation with mitotic exit and the activation of APC/CCdh1. Further, we uncover differential proteolysis rates for E2F8 at different points within G1 phase, accounting for its accumulation in late G1 while APC/CCdh1 is still active. Finally, we demonstrate that the F-box protein Cyclin F regulates E2F8 in G2-phase. Altogether, our data define E2F8 regulation throughout the cell cycle, illuminating an extensive coordination between phosphorylation, ubiquitination and transcription in mammalian cell cycle.
Figures






References
-
- Ayad NG, Rankin S, Murakami M, Jebanathirajah J, Gygi S, Kirschner MW. (2003). Tome-1, a trigger of mitotic entry, is degraded during G1 via the APC. Cell , 101–113. - PubMed
-
- Ayad NG, Rankin S, Ooi D, Rape M, Kirschner MW. (2005). Identification of ubiquitin ligase substrates by in vitro expression cloning. Methods Enzymol , 404–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

