Indirect costs of adult pneumococcal disease and the productivity-based rate of return to the 13-valent pneumococcal conjugate vaccine for adults in Turkey
- PMID: 31995443
- PMCID: PMC7482724
- DOI: 10.1080/21645515.2019.1708668
Indirect costs of adult pneumococcal disease and the productivity-based rate of return to the 13-valent pneumococcal conjugate vaccine for adults in Turkey
Abstract
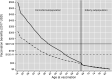
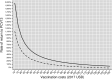
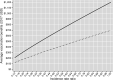
Productivity benefits of health technologies are ignored in typical economic evaluations from a health payer's perspective, risking undervaluation. We conduct a productivity-based cost-benefit analysis from a societal perspective and estimate indirect costs of adult pneumococcal disease, vaccination benefits from the adult 13-valent pneumococcal conjugate vaccine (PCV13 Adult), and rates of return to PCV13 Adult for a range of hypothetical vaccination costs. Our context is Turkey's funding PCV13 for the elderly and for non-elderly adults with select comorbidities within the Ministry of Health's National Immunization Program. We use a Markov model with one-year cycles. Indirect costs from death or disability equal the expected present discounted value of lifetime losses in the infected individual's paid and unpaid work and in caregivers' paid work. Vaccination benefits comprise averted indirect costs. Rates of return equal vaccination benefits divided by vaccination costs, minus one. Input parameters are from public data sources. We model comorbidities' effects by scalar multiplication of the parameters of the general population. Indirect costs per treatment episode of inpatient community-acquired pneumonia (CAP), bacteremia, and meningitis - but not for outpatient CAP - approach or exceed Turkish per capita gross domestic product. Vaccination benefits equal $207.02 per vaccination in 2017 US dollars. The rate of return is positive for all hypothetical costs below this. Results are sensitive to herd effects from pediatric vaccination and vaccine efficacy rates. For a wide range of hypothetical vaccination costs, the rate of return compares favorably with those of other global development interventions with well-established strong investment cases.
Keywords: PCV13 Adult; Turkey; cost-benefit analysis; economic evaluation; indirect costs; social rate of return; vaccines.
Conflict of interest statement
This study was led by Data for Decisions, LLC (DfD) and sponsored by Pfizer. JP Sevilla and D. Burnes are employees of DfD. A. Stawasz and A. Agarwal were employees of DfD. D. Bloom is a paid consultant to DfD. R. Sato, B. Hacibedel, and K. Helvacıoğlu are employees of Pfizer Inc. The sponsor had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures

References
-
- Plotkin SL, Plotkin SA.. A short history of vaccination. In: Plotkin SA, Orenstein WA, editors. Vaccines. 4th ed. Philadelphia (PA): W.B. Saunders Company; 2004. p. 1–15.
-
- Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, editors. Cost-effectiveness in health and medicine. 2nd ed. New York (NY): Oxford University Press; 2017.
-
- Bleichrodt H, Quiggin J.. Life-cycle preferences over consumption and health: when is cost-effectiveness analysis equivalent to cost-benefit analysis? J Health Econ. 1999;18:681–708. - PubMed
-
- Hammitt J. Admissible utility functions for health, longevity, and wealth: integrating monetary and life-year measures. J Risk Uncertain. 2013;47:311–25.
-
- Brouwer WB, Koopmanschap MA, Rutten FF. Productivity costs in cost-effectiveness analysis: numerator or denominator: a further discussion. Health Econ. 1997;6:511–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
