HIV/HCV therapy with ledipasvir/sofosbuvir after randomized switch to emtricitabine-tenofovir alafenamide-based single-tablet regimens
- PMID: 31995556
- PMCID: PMC6988963
- DOI: 10.1371/journal.pone.0224875
HIV/HCV therapy with ledipasvir/sofosbuvir after randomized switch to emtricitabine-tenofovir alafenamide-based single-tablet regimens
Abstract
Introduction: Guidelines advocate the treatment of HCV in all HIV/HCV co-infected individuals. The aim of this randomized, open-label study (ClinicalTrials.gov identifier: NCT02707601; https://clinicaltrials.gov/ct2/show/NCT02707601) was to evaluate the safety/efficacy of ledipasvir/sofosbuvir (LDV/SOF) co-administered with elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) or rilpivirine/F/TAF (R/F/TAF) in HIV-1/HCV co-infected participants.
Methods: Participants with HIV-1 RNA <50 copies/mL and chronic HCV-genotype (GT) 1 (HCV treatment-naïve ± compensated cirrhosis or HCV treatment-experienced non-cirrhotic) were randomized 1:1 to switch to E/C/F/TAF or R/F/TAF. If HIV suppression was maintained at Week 8, participants received 12 weeks of LDV/SOF. The primary endpoint was sustained HCV virologic response 12 weeks after LDV/SOF completion (SVR12).
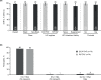
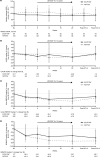
Results: Of 150 participants, 148 received ≥1 dose of HIV study drug and 144 received LDV/SOF (72 in each F/TAF group; 83% GT1a, 94% HCV treatment-naïve, 12% cirrhotic). Overall, SVR12 was 97% (95% confidence interval: 93-99%). Black race did not affect SVR12. Of four participants not achieving SVR12, one had HCV relapse, one had HCV virologic non-response due to non-adherence, and two missed the post-HCV Week 12 visit. Of 148 participants, 96% receiving E/C/F/TAF and 95% receiving R/F/TAF maintained HIV suppression at Week 24; no HIV resistance was detected. No participant discontinued LDV/SOF or E/C/F/TAF due to adverse events; one participant discontinued R/F/TAF due to worsening of pre-existing hypercholesterolemia. Renal toxicity was not observed in either F/TAF regimen during LDV/SOF co-administration. In conclusion, high rates of HCV SVR12 and maintenance of HIV suppression were achieved with LDV/SOF and F/TAF-based regimens.
Conclusion: This study supports LDV/SOF co-administered with an F/TAF-based regimen in HIV-1/HCV-GT1 co-infected patients.
Conflict of interest statement
GH has received grants from Gilead, ViiV Healthcare, Janssen and Proteus and has been a scientific advisor for Gilead, ViiV Healthcare, Janssen and Theratechnologies. MR is a speaker for Gilead, Janssen, and AbbVie and has participated in advisory boards/has consulted for Gilead and Merck. MJ receives research funding from Gilead, Janssen, Merck, and GSK/ViiV Healthcare, and has participated in advisory boards for GSK. FH has received grant/research support from AbbVie, Gilead, and Janssen, and has participated in sponsored lectures for AbbVie, Gilead, Janssen, and Merck. DA has participated on a speaker board for Gilead, Merck, and Janssen as well as advisory boards for Gilead, GSK/ViiV Healthcare, and Napo Pharmaceuticals. JS has participated in speaker bureaus for Gilead, Merck, AbbVie, and Janssen. DG participated in an advisory board for Gilead. SA is an advisor and speaker for Gilead and Merck. JR, SJ, SC, MD, DP, BG, LR, and RH are employees of Gilead and hold stock interests in the company. TN-C was an employee at Gilead Sciences during the conduct of the study. He is currently an employee at Arena Pharmaceuticals, San Diego, CA, USA. The study was funded by Gilead Sciences, Inc., Foster City, CA, USA. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Ledipasvir/sofosbuvir for treatment of hepatitis C virus in sofosbuvir-experienced, NS5A treatment-naïve patients: Findings from two randomized trials.Liver Int. 2018 Jun;38(6):1010-1021. doi: 10.1111/liv.13616. Epub 2017 Dec 5. Liver Int. 2018. PMID: 29091342 Free PMC article. Clinical Trial.
-
Hepatitis C virus treatment response to ledipasvir/sofosbuvir among patients coinfected with HIV and HCV: Real world data in a black population.Medicine (Baltimore). 2020 Mar;99(11):e19140. doi: 10.1097/MD.0000000000019140. Medicine (Baltimore). 2020. PMID: 32176039 Free PMC article.
-
Effectiveness of All-Oral Antiviral Regimens in 996 Human Immunodeficiency Virus/Hepatitis C Virus Genotype 1-Coinfected Patients Treated in Routine Practice.Clin Infect Dis. 2017 Jun 15;64(12):1711-1720. doi: 10.1093/cid/cix111. Clin Infect Dis. 2017. PMID: 28199525
-
Fixed-dose combination of sofosbuvir and ledipasvir for the treatment of chronic hepatitis C genotype 1.Expert Opin Pharmacother. 2015 Apr;16(5):739-48. doi: 10.1517/14656566.2015.1013938. Epub 2015 Feb 13. Expert Opin Pharmacother. 2015. PMID: 25676581 Review.
-
Efficacy and safety of ledipasvir/sofosbuvir for hepatitis C among drug users: a systematic review and meta-analysis.Virol J. 2021 Jul 27;18(1):156. doi: 10.1186/s12985-021-01625-w. Virol J. 2021. PMID: 34315488 Free PMC article.
Cited by
-
Drug combination therapy for emerging viral diseases.Drug Discov Today. 2021 Oct;26(10):2367-2376. doi: 10.1016/j.drudis.2021.05.008. Epub 2021 May 21. Drug Discov Today. 2021. PMID: 34023496 Free PMC article. Review.
-
Sustained virological response after treatment with direct-acting antivirals can help immune reconstitution in HIV-HCV coinfected patients even in case of persistent HIV low-level viremia.Health Sci Rep. 2020 Dec 21;4(1):e221. doi: 10.1002/hsr2.221. eCollection 2021 Mar. Health Sci Rep. 2020. PMID: 33364441 Free PMC article. No abstract available.
References
-
- The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. Available from: https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVG... Cited 7 March 2019. - PMC - PubMed
-
- European AIDS Clinical Society. EACS Guidelines version 9.0 October 2017. Available from: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html Cited 7 March 2019.
-
- Jain MK, Chavez C, Sanders J, Vysyaraju K. Hepatitis C eradication: who is being left behind in the HIV population? IDWeek 2018, October 3–7, 2018, San Francisco Abstract 2233 Poster available at: https://idsaconfexcom/idsa/2018/webprogram/Paper70237html.
-
- Jayaweera D, Althoff K, Eron JJ, Huhn G, Milligan S, Mills A, et al. Untreated HCV in HIV/HCV co-infection; data from the Trio Network. Gastroenterology. 2018; 154(6): S-1189 10.1016/S0016-5085(18)33931-3 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

