Comparative specificity and sensitivity of NS1-based serological assays for the detection of flavivirus immune response
- PMID: 31995566
- PMCID: PMC7010293
- DOI: 10.1371/journal.pntd.0008039
Comparative specificity and sensitivity of NS1-based serological assays for the detection of flavivirus immune response
Abstract
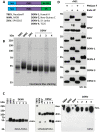
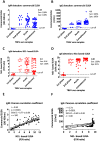
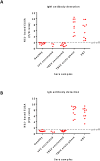
Flaviviruses are relevant animal and human pathogens of increasing importance worldwide. The similarities of the initial clinical symptoms and the serological cross-reactivity of viral structural antigens make a laboratory diagnosis of flavivirus infection problematic. The main aim of the present study was the comparative specificity and sensitivity analysis of the non-structural protein NS1 as an antigen to detect flavivirus antibodies in sera from exposed individuals. A strategy for the purification of native recombinant non-structural protein 1 of representative flaviviruses including tick-borne encephalitis, West Nile, Zika and dengue virus was developed. The immunological properties of the purified antigens were analyzed using sera of immunized mice and of infected individuals in comparison with standard commercial assays. Recombinant NS1 protein was confirmed as a valuable option for the detection of flavivirus antibodies with reduced cross-reactivity and high sensitivity offering additional advantages for the detection of vaccine breakthrough cases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Williams DT, Mackenzie JS, Bingham J. Flaviviruses In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J, editors. Diseases of swine: John Wiley & Sons, Inc; 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

