Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants
- PMID: 31995650
- PMCID: PMC6988992
- DOI: 10.1002/14651858.CD000361.pub4
Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants
Abstract
Background: Nosocomial infections continue to be a significant cause of morbidity and mortality among preterm and/or low birth weight (LBW) infants. Preterm infants are deficient in immunoglobulin G (IgG); therefore, administration of intravenous immunoglobulin (IVIG) may have the potential of preventing or altering the course of nosocomial infections.
Objectives: To use systematic review/meta-analytical techniques to determine whether IVIG administration (compared with placebo or no intervention) to preterm (< 37 weeks' postmenstrual age (PMA) at birth) or LBW (< 2500 g birth weight) infants or both is effective/safe in preventing nosocomial infection.
Search methods: For this update, MEDLINE, EMBASE, CINAHL, The Cochrane Library, Controlled Trials, ClinicalTrials.gov and PAS Abstracts2view were searched in May 2013.
Selection criteria: We selected randomised controlled trials (RCTs) in which a group of participants to whom IVIG was given was compared with a control group that received a placebo or no intervention for preterm (< 37 weeks' gestational age) and/or LBW (< 2500 g) infants. Studies that were primarily designed to assess the effect of IVIG on humoral immune markers were excluded, as were studies in which the follow-up period was one week or less.
Data collection and analysis: Data collection and analysis was performed in accordance with the methods of the Cochrane Neonatal Review Group.
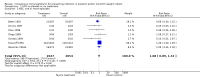
Main results: Nineteen studies enrolling approximately 5000 preterm and/or LBW infants met inclusion criteria. No new trials were identified in May 2013. When all studies were combined, a significant reduction in sepsis was noted (typical risk ratio (RR) 0.85, 95% confidence interval (CI) 0.74 to 0.98; typical risk difference (RD) -0.03, 95% CI 0.00 to -0.05; number needed to treat for an additional beneficial outcome (NNTB) 33, 95% CI 20 to infinity), and moderate between-study heterogeneity was reported (I2 54% for RR, 55% for RD). A significant reduction of one or more episodes was found for any serious infection when all studies were combined (typical RR 0.82, 95% CI 0.74 to 0.92; typical RD -0.04, 95% CI -0.02 to -0.06; NNTB 25, 95% CI 17 to 50), and moderate between-study heterogeneity was observed (I2 50% for RR, 62% for RD). No statistically significant differences in mortality from all causes were noted (typical RR 0.89, 95% CI 0.75 to 1.05; typical RD -0.01, 95% CI -0.03 to 0.01), and no heterogeneity for RR (I2 = 21%) or low heterogeneity for RD was documented (I2 = 28%). No statistically significant difference was seen in mortality from infection; in incidence of necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD) or intraventricular haemorrhage (IVH) or in length of hospital stay. No major adverse effects of IVIG were reported in any of these studies.
Authors' conclusions: IVIG administration results in a 3% reduction in sepsis and a 4% reduction in one or more episodes of any serious infection but is not associated with reductions in other clinically important outcomes, including mortality. Prophylactic use of IVIG is not associated with any short-term serious side effects. The decision to use prophylactic IVIG will depend on the costs and the values assigned to the clinical outcomes. There is no justification for conducting additional RCTs to test the efficacy of previously studied IVIG preparations in reducing nosocomial infections in preterm and/or LBW infants.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
None
Figures











Update of
-
Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants.Cochrane Database Syst Rev. 2013 Jul 2;(7):CD000361. doi: 10.1002/14651858.CD000361.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Jan 29;1:CD000361. doi: 10.1002/14651858.CD000361.pub4. PMID: 23821390 Updated.
References
References to studies included in this review
Atici 1996 {published data only}
-
- Atici A, Satar M, Karabay A, Yilimaz M. Intravenous immunoglobulin for prophylaxis of nosocomial sepsis. Indian Journal of Pediatrics 1996;63:517‐21. - PubMed
Baker 1992 {published data only}
-
- Baker CJ, Melish ME, Hall RT, et al. Intravenous immune globulin for the prevention of nosocomial infection in low‐birth‐weight neonates. New England Journal of Medicine 1992;327:213‐9. - PubMed
Bussel 1990a {published data only}
-
- Bussel JB. Intravenous gammaglobulin in the prophylaxis of late sepsis in very‐low‐birth‐weight infants: preliminary results of a randomized, double‐blind, placebo‐controlled trial. Reviews of Infectious Diseases 1990;12:S457‐62. - PubMed
Chirico 1987 {published data only}
-
- Chirico G, Rondini G, Plebani A, Chiara A, Massa M, Ugazio AG. Intravenous gammaglobulin therapy for prophylaxis of infection in high‐risk neonates. Journal of Pediatrics 1987;110:437‐42. - PubMed
Chou 1998 {published data only}
-
- Chou Y‐H, Yau K‐I T. The use of prophylactic intravenous immunoglobulin therapy in very low birthweight infants. Chang Gung Medical Journal 1998;21:371‐6. - PubMed
Christensen 1989 {published data only}
-
- Christensen RD, Hardman T, Thornton J, Hill HR. A randomized, double‐blind, placebo‐controlled investigation of the safety of intravenous immune globulin administration to preterm neonates. Journal of Perinatology 1989;9:126‐30. - PubMed
Clapp 1989 {published data only}
-
- Clapp DW, Kliegman RM, Baley JE, et al. Use of intravenously administered immune globulin to prevent nosocomial sepsis in low birth weight infants: report of a pilot study. Journal of Pediatrics 1989;115:973‐8. - PubMed
Conway 1990 {published data only}
-
- Conway SP, Ng PC, Howel D, Maclain B, Gooi HC. Prophylactic intravenous immunoglobulin in pre‐term infants: a controlled trial. Vox Sanguinis 1990;59:6‐11. - PubMed
Didato 1988 {published data only}
-
- Didato MA, Gioeli R, Priolisi A. The use of intravenous gamma‐globulin for prevention of sepsis in pre‐term infants. Helvetica Paediatrica Acta 1988;43:283‐94. - PubMed
Fanaroff 1994 {published data only}
-
- Fanaroff AA, Korones SB, Wright LL, et al. A controlled trial of intravenous immune globulin to reduce nosocomial infections in very‐low‐birth‐weight infants. New England Journal of Medicine 1994;330:1107‐13. - PubMed
Fanaroff‐I 1994 {published data only}
-
- Fanaroff‐1. Phase 1 of Fanaroff 1994. New England Journal of Medicine 1994; Vol. 330:1107‐13.
Haque 1986 {published data only}
-
- Haque KN, Zaidi MH, Haque SK, Bahakim H, El‐Hazmi M, El‐Swailam M. Intravenous immunoglobulin for prevention of sepsis in preterm and low birth weight infants. Pediatric Infectious Disease 1986;5:622‐5. - PubMed
Magny 1991b {published data only}
-
- Magny JF, Bremard‐Oury C, Brault D, Menguy C, Voyer M, Landais P, et al. Intravenous immunoglobulin therapy for prevention of infection in high‐risk premature infants: report of a multicenter, double‐blind study. Pediatrics 1991;88:437‐43. - PubMed
Ratrisawadi 1991 {published data only}
-
- Ratrisawadi V, Srisuwanporn T, Puapondh Y. Intravenous immunoglobulin prophylaxis for infection in very low birth‐weight infants. Journal of the Medical Association of Thailand 1991;74:14‐8. - PubMed
Sandberg 2000 {published data only}
-
- Sandberg K, Fasth A, Berger A, Eibl M, Isacson K, Lisebka A, et al. Preterm infants with low immunoglobulin G levels have increased risk of neonatal sepsis but do not benefit from prophylactic immunoglobulin G. Journal of Pediatrics 2000;137:623‐8. - PubMed
Spady 1994 {published data only}
-
- Spady DW, Pabst HF, Byrnes P. Intravenous immunoglobulin (IVIG) shortens stay for low birth weight infants [abstract]. Pediatr Research 1994;35:304A.
Stabile 1988 {published data only}
Tanzer 1997 {published data only}
-
- Tanzer F, Yazar N, Hakgudener Y, Kafali G. Intravenous immunoglobulin for sepsis prevention in preterm infants. Turkish Journal of Pediatrics 1997;39:341‐5. - PubMed
Van Overmeire 1993 {published data only}
-
- Overmeire B, Bleyaert S, Reempts PJ, Acker KJ. The use of intravenously administered immunoglobulins in the prevention of severe infection in very low birth weight neonates. Biology of the Neonate 1993;64:110‐5. - PubMed
Weisman 1994a {published data only}
-
- Weisman LE, Stoll BJ, Kueser TJ, et al. Intravenous immune globulin prophylaxis of late‐onset sepsis in premature neonates. Journal of Pediatrics 1994;125:922‐30. - PubMed
References to studies excluded from this review
Acunas 1994 {published data only}
Adhikari 1996 {published data only}
-
- Adhikari M, Wesley AG, Fourie PB. Intravenous immunoglobulin prophylaxis in neonates on artificial ventilation. South African Medical Journal 1996;86:542‐5. - PubMed
Kacet 1991 {published data only}
-
- Kacet N, Gremillet C, Zaoui C, et al. Prevention of late‐onset infections in preterm infants with intravenous gamma‐globulin: a randomized clinical trial [abstract ]. European Journal of Pediatrics 1991;150:604.
Kinney 1991 {published data only}
-
- Kinney J, Mundorf L, Gleason C, et al. Efficacy and pharmacokinetics of intravenous immune globulin administration to high‐risk neonates. American Journal of Diseases of Children 1991;145:1233‐8. - PubMed
Lelik 2004 {published data only}
-
- Lelik MP, Efanova EA. Prevention of nosocomial infections in newborns at artificial lung ventilation. Anesteziologiia i Reanimatologiia 2004;May‐June(3):41‐3. - PubMed
Malik 1990 {published data only}
-
- Malik S, Giacoia GP, West K, Miller G. Intravenous immunoglobulin (IVIG) to prevent infections in infants with bronchopulmonary dysplasia (BPD) [abstract]. Pediatric Research 1990;27:273A.
Monintja 1989 {published data only}
-
- Monintja HE. Investigation on immunoglobulin fortification in preventing infections in the newborn. Paediatrica Indonesiana 1989;29:91‐6. - PubMed
Additional references
Baker 1990a
-
- Baker CJ. New uses of intravenous immune globulin in newborn infants. Journal of Clinical Immunology 1990;10:47S‐55S. - PubMed
Baker 1990b
-
- Baker CJ, Rench MA, Noya FJD, Garcia‐Prats JA, and the Neonatal IVIG Study Group. Role of intravenous immunoglobulin in prevention of late‐onset infection in low‐birth‐weight neonates. Reviews of Infectious Diseases 1990;12:S463‐9. - PubMed
Baley 1988
-
- Baley JE. Neonatal sepsis: the potential for immunotherapy. Clinics in Perinatology 1988;15:755‐71. - PubMed
Baley 1992
-
- Baley JE, Fanaroff AA. Neonatal infections. Part 2: Specific infectious diseases and therapies. In: Sinclair J, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:496‐506.
Bell 1978
Berger 1991
-
- Berger M. Use of intravenously administered immune globulin in newborn infants: prophylaxis, treatment, both, or neither. Journal of Pediatrics 1991;118:557‐9. - PubMed
Bortolussi 1986a
-
- Bortolussi R, Fischer GW. Opsonic and protective activity of immunoglobulin, modified immunoglobulin, and serum against neonatal Escherichia coli K1 infection. Pediatric Research 1986;20:175‐8. - PubMed
Bortolussi 1986b
-
- Bortolussi R. Potential for intravenous gamma‐globulin use in neonatal gram‐negative infection: an overview. Pediatric Infectious Diseases 1986;5:S198‐200. - PubMed
Bussel 1990b
-
- Bussel JB. Neonatal uses of intravenous immunoglobulin. American Journal of Pediatric Hematol/Oncology 1990;12:505‐9. - PubMed
Consensus 1997
-
- Consensus Working Group. Present and future uses of IVIG: a Canadian multidisciplinary consensus‐building initiative. Canadian Journal of Allergy and Clinical Immunology 1997;2:176‐208.
Fischer 1986
-
- Fischer GW, Hemming VG, Hunter KW, et al. Intravenous immunoglobulin in the treatment of neonatal sepsis: therapeutic strategies and laboratory studies. Pediatric Infectious Disease 1986;5:S171‐5. - PubMed
Fischer 1988
-
- Fischer GW. Therapeutic uses of intravenous gammaglobulin for pediatric infections. Pediatric Clinics of North America 1988;35:517‐33. - PubMed
Fischer 1990a
-
- Fischer GW, Weisman LE. Therapeutic intervention of clinical sepsis with intravenous immunoglobulin, white blood cells and antibiotics. Scandinavian Journal of Infectious Diseases 1990;73 Suppl:17‐21. - PubMed
Fischer 1990b
-
- Fischer GW, Hemming VG, Gloser HP, Bachmyer H, Pilar CE, Wilson SR, et al. Polyvalent group B streptococcal immune globulin for intravenous administration: overview. Reviews of Infectious Diseases 1990;12:S483‐91. - PubMed
Fischer 1990c
-
- Fischer GW. Immunoglobulin therapy for neonatal sepsis: an overview of animal and clinical studies. Journal of Clinical Immunology 1990;10:40S‐6S. - PubMed
Gonzalez 1989
-
- Gonzalez LA, Hill HR. The current status of intravenous gamma‐globulin use in neonates. Pediatric Infectious Disease Journal 1989;8:315‐22. - PubMed
Hammarstrom 1990
-
- Hammarstrom L, Smith CIE. The use of intravenous IgG as prophylaxis and for treatment of infections. Infection 1990;18:314‐24. - PubMed
Haque 1992
-
- Haque KH. Does the commercial type of IVIG used make a difference?. Pediatrics 1992;89:806‐7. - PubMed
Higgins 2003
Hill 1993
-
- Hill HR. Intravenous immunoglobulin use in the neonate: role in prophylaxis and therapy of infection. Pediatric Infectious Disease Journal 1993;12:549‐59. - PubMed
Hill 1991a
-
- Hill RH. Is prophylaxis of neonates with intravenous immunoglobulin beneficial. American Journal of Diseases of Children 1991;145:1229‐30. - PubMed
Hill 1991b
-
- Hill HR. The role of intravenous immunoglobulin in the treatment and prevention of neonatal bacterial infection. Seminars in Perinatology 1991;15:41‐6. - PubMed
Hobbs 1967
-
- Hobbs JR, Davis JA. Serum IgG‐globulin levels and gestational age in premature babies. Lancet 1967;I:757‐9. - PubMed
Irani 1991
-
- Irani SF, Wagle SU, Deshpande PG. Role of intravenous immunoglobulin in prevention and treatment of neonatal infection. Indian Pediatrics 1991;28:443‐9. - PubMed
Jenson 1997
-
- Jenson HB, Pollock BH. Meta‐analyses of the effectiveness of intravenous immune globulin for prevention and treatment of neonatal sepsis. Pediatrics 1997;99:e2. - PubMed
Johnston 1990
-
- Johnston RB. Immunotherapy and immunoprophylaxis in the newborn infant: the need for definitive trials. Reviews of Infectious Diseases 1990;12:S392‐3. - PubMed
Kliegman 1990
-
- Kliegman RM, Clapp DW, Berger M. Targeted immunoglobulin therapy for the prevention of neonatal infections. Reviews of Infectious Diseases 1990;12:S443‐56. - PubMed
Kliegman 1991
-
- Kliegman RM, Clapp DW. Rational principles for immunoglobulin prophylaxis and therapy for neonatal infections. Clinics in Perinatology 1991;18:303‐324. - PubMed
Kyllonen 1989
-
- Kyllonen KS, Clapp W, Kliegman RM, Baley JE, Shenker N, Fanaroff AA, et al. Dosage of intravenously administered immune globulin and dosing interval required to maintain target levels of immunoglobulin G in low birth weight infants. Journal of Pediatrics 1989;115:1013‐6. - PubMed
Lacy 1995
Magny 1991a
-
- Magny J‐F. Les immunoglobulines sont‐elles utiles dans le traitment des infections neonatales?. La Revue du Praticien 1991;41:1368‐70. - PubMed
Noya 1989
-
- Noya FJD, Baker CJ. Intravenously administered immune globulin for premature infants: a time to wait. Journal of Pediatrics 1989;115:969‐71. - PubMed
Papile 1983
-
- Papile LA, Munsick‐Bruno G, Schafer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. Journal of Pediatrics 1983;103:273‐7. - PubMed
Rondini 1991
-
- Rondini G, Chirico G, Ugazio AG. Intravenous immunoglobulin for prophylaxis of infection in preterm infants. Developmental Pharmacology and Therapeutics 1991;17:144‐9. - PubMed
Salzer 1991
-
- Salzer HR, Weninger M, Pollak A, Kolmer M. Prophylactic immunoglobulin (IG) treatment in infants less than 30 weeks gestation-a meta analysis [abstract]. European Journal of Pediatrics 1991;150:604.
Siber 1992
-
- Siber GR. Immune globulin to prevent nosocomial infections. New England Journal of Medicine 1992;327:269‐71. - PubMed
Stabile 1989
-
- Stabile A, Sopo SM, Pastore M, Pesaresi MA. Intravenous immune globulin doses and infection prophylaxis in very low birth weight neonates. Journal of Pediatrics 1989;114:168. - PubMed
Stiehm 1966
-
- Stiehm RE, Fudenberg HH. Serum levels of immune globulins in health and disease: a survey. Pediatrics 1966;37:715‐27. - PubMed
Stiehm 1986
-
- Stiehm ER. Intravenous immunoglobulins in neonates and infants: an overview. Pediatric Infectious Disease 1986;5:S217‐9. - PubMed
Stiehm 1990
-
- Stiehm ER. Role of immunoglobulin therapy in neonatal infections: where we stand today. Reviews of Infectious Diseases 1990;12:S439‐42. - PubMed
Stoll 1996
-
- Stoll BJ, Gordon T, Korones SB, et al. Late‐onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. Journal of Pediatrics 1996;129:63‐71. - PubMed
Weisman 1986
-
- Weisman LE, Fischer GW, Hemming VG, Peck CC. Pharmacokinetics of intravenous immunoglobulin (Sandoglobulin) in neonates. Pediatric Infectious Disease 1986;5:S185‐8. - PubMed
Weisman 1992
-
- Weisman LE, Cruess DF, Fischer GW. Current status of intravenous immunoglobulin in preventing or treating neonatal bacterial infections. Clinical Reviews in Allergy 1992;10:13‐28. - PubMed
Weisman 1993
-
- Weisman LE, Cruess DF, Fischer GW. Standard versus hyperimmune immunoglobulin in preventing or treating neonatal bacterial infections. Clinics in Perinatology 1993;20:211‐24. - PubMed
Weisman 1994b
-
- Weisman LE, Cruess DF, Fischer GW. Opsonic activity of commercially available standard intravenous immunoglobulin preparations. Pediatric Infectious Disease Journal 1994;13:1122‐5. - PubMed
Whitelaw 1990
Wolach 1997
-
- Wolach B. Neonatal sepsis: pathogenesis and supportive therapy. Seminars in Perinatology 1997;21:28‐38. - PubMed
References to other published versions of this review
Ohlsson 1998
-
- Ohlsson A, Lacy JB. Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000361] - DOI
Ohlsson 2001
Ohlsson 2004
Ohlsson 2007
-
- Ohlsson A, Lacy JB. Intravenous immunoglobulin for preventing infection in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000361] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

