Differential roles and activation of mammalian target of rapamycin complexes 1 and 2 during cell migration in prostate cancer cells
- PMID: 31995655
- PMCID: PMC7232714
- DOI: 10.1002/pros.23956
Differential roles and activation of mammalian target of rapamycin complexes 1 and 2 during cell migration in prostate cancer cells
Abstract
Background: Mammalian target of rapamycin (mTOR) is a downstream substrate activated by PI3K/AKT pathway and it is essential for cell migration. It exists as two complexes: mTORC1 and mTORC2. mTORC1 is known to be regulated by active AKT, but the activation of mTORC2 is poorly understood. In this study, we investigated the roles and differential activation of the two mTOR complexes during cell migration in prostate cancer cells.
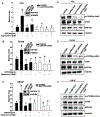
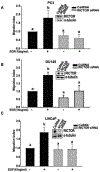
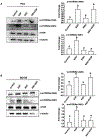
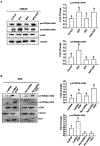
Methods: We used small interfering RNA to silence the expression of Rac1 and the main components of mTOR complexes (regulatory associated protein of mTOR [RAPTOR] and rapamycin-insensitive companion of mTOR [RICTOR]) in LNCaP, DU145, and PC3 prostate cancer cell lines. We performed transwell migration assay to evaluate the migratory capability of the cells, and Western blot analysis to study the activation levels of mTOR complexes.
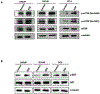
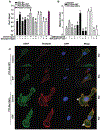
Results: Specific knockdown of RAPTOR and RICTOR caused a decrease of cell migration, suggesting their essential role in prostate cancer cell movement. Furthermore, epidermal growth factor (EGF) treatments induced the activation of both the mTOR complexes. Lack of Rac1 activity in prostate cancer cells blocked EGF-induced activation of mTORC2, but had no effect on mTORC1 activation. Furthermore, the overexpression of constitutively active Rac1 resulted in significant increase in cell migration and activation of mTORC2 in PC3 cells, but had no effect on mTORC1 activation. Active Rac1 was localized in the plasma membrane and was found to be in a protein complex, with RICTOR, but not RAPTOR.
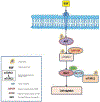
Conclusion: We suggest that EGF-induced activation of Rac1 causes the activation of mTORC2 via RICTOR. This mechanism plays a critical role in prostate cancer cell migration.
Keywords: PI3K/AKT/mTOR; Rac1; cell migration; mTORC1; mTORC2; prostate cancer.
© 2020 Wiley Periodicals, Inc.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

