Using Real-World Data to Guide Ustekinumab Dosing Strategies for Psoriasis: A Prospective Pharmacokinetic-Pharmacodynamic Study
- PMID: 31995663
- PMCID: PMC7070790
- DOI: 10.1111/cts.12725
Using Real-World Data to Guide Ustekinumab Dosing Strategies for Psoriasis: A Prospective Pharmacokinetic-Pharmacodynamic Study
Abstract
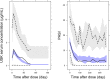
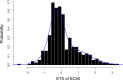
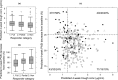
Variation in response to biologic therapy for inflammatory diseases, such as psoriasis, is partly driven by variation in drug exposure. Real-world psoriasis data were used to develop a pharmacokinetic/pharmacodynamic (PK/PD) model for the first-line therapeutic antibody ustekinumab. The impact of differing dosing strategies on response was explored. Data were collected from a UK prospective multicenter observational cohort (491 patients on ustekinumab monotherapy, drug levels, and anti-drug antibody measurements on 797 serum samples, 1,590 measurements of Psoriasis Area Severity Index (PASI)). Ustekinumab PKs were described with a linear one-compartment model. A maximum effect (Emax ) model inhibited progression of psoriatic skin lesions in the turnover PD mechanism describing PASI evolution while on treatment. A mixture model on half-maximal effective concentration identified a potential nonresponder group, with simulations suggesting that, in future, the model could be incorporated into a Bayesian therapeutic drug monitoring "dashboard" to individualize dosing and improve treatment outcomes.
© 2019 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
C.E.M.G. has received honoraria and/or research grant support (University of Manchester) from AbbVie, Almirall, Bristol Meyers Squibb, Celgene, GSK, Janssen, LEO Foundation, Lilly, Novartis, Pfizer, Sandoz, Sun Pharma, and UCB Pharma. N.J.R. has received honoraria, travel support, and/or research grants (Newcastle University) from AbbVie, Almirall, Celgene, Genentech, Janssen, Novartis, Pfizer, Sanofi Genzyme Regeneron, and UCB Pharma Ltd. J.B. has received honoraria, travel support, and/or research grants (King's College) from AbbVie, Pfizer, Novartis, Janssen, Roche, Regeneron, Lilly, UCB, Sun Pharma, Boehringer Ingelheim, and GSK. R.B.W. has received honoraria and/or research grants from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen, Leo, Lilly, Novartis, Pfizer, Sanofi, Xenoport, and UCB. A.D.B. has received honoraria from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, Leo, Lilly, Novartis, and Pfizer. T.R. has received honoraria for lectures from Pfizer, AbbVie, and Regeneron, and a research grant from Genmab. D.S. has received departmental research funding from AstraZeneca. C.S. has received departmental research funding from AbbVie, GSK, Pfizer, Novartis, Regeneron, and Roche. N.W. acts as statistician on a trial funded by AstraZeneca. The PSORT consortium has a number of industry partners; see
Figures

References
-
- Warren, R.B. et al Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J. Invest. Dermatol. 135, 2632–2640 (2015). - PubMed
-
- Favalli, E.G. , Raimondo, M.G. , Becciolini, A. , Crotti, C. , Biggioggero, M. & Caporali, R. The management of first‐line biologic therapy failures in rheumatoid arthritis: current practice and future perspectives. Autoimmun. Rev. 16, 1185–1195 (2017). - PubMed
-
- Yanai, H. & Hanauer, S.B. Assessing response and loss of response to biological therapies in IBD. Am. J. Gastroenterol. 106, 685–698 (2011). - PubMed
-
- Krieckaert, C.L.M. et al Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann. Rheum. Dis. 74, 361–368 (2015). - PubMed
-
- Yarur, A.J. et al Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn's disease and ulcerative colitis. Inflamm. Bowel Dis. 22, 409–415 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

