Nonspherical Coacervate Shapes in an Enzyme-Driven Active System
- PMID: 31995710
- PMCID: PMC7057537
- DOI: 10.1021/acs.langmuir.9b02719
Nonspherical Coacervate Shapes in an Enzyme-Driven Active System
Abstract
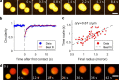
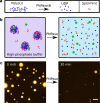
Coacervates are polymer-rich droplets that form through liquid-liquid phase separation in polymer solutions. Liquid-liquid phase separation and coacervation have recently been shown to play an important role in the organization of biological systems. Such systems are highly dynamic and under continuous influence of enzymatic and chemical processes. However, it is still unclear how enzymatic and chemical reactions affect the coacervation process. Here, we present and characterize a system of enzymatically active coacervates containing spermine, RNA, free nucleotides, and the template independent RNA (de)polymerase PNPase. We find that these RNA coacervates display transient nonspherical shapes, and we systematically study how PNPase concentration, UDP concentration, and temperature affect coacervate morphology. Furthermore, we show that PNPase localizes predominantly into the coacervate phase and that its depolymerization activity in high-phosphate buffer causes coacervate degradation. Our observations of nonspherical coacervate shapes may have broader implications for the relationship between (bio)chemical activity and coacervate biology.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Impact of macromolecular crowding on RNA/spermine complex coacervation and oligonucleotide compartmentalization.Soft Matter. 2018 Jan 17;14(3):368-378. doi: 10.1039/c7sm02146a. Soft Matter. 2018. PMID: 29265152
-
How Droplets Can Accelerate Reactions─Coacervate Protocells as Catalytic Microcompartments.Acc Chem Res. 2024 Jul 16;57(14):1885-1895. doi: 10.1021/acs.accounts.4c00114. Epub 2024 Jul 5. Acc Chem Res. 2024. PMID: 38968602 Free PMC article.
-
Reversible pH-Responsive Coacervate Formation in Lipid Vesicles Activates Dormant Enzymatic Reactions.Angew Chem Int Ed Engl. 2020 Apr 6;59(15):5950-5957. doi: 10.1002/anie.201914893. Epub 2020 Feb 26. Angew Chem Int Ed Engl. 2020. PMID: 31943629 Free PMC article.
-
Form Equals Function: Influence of Coacervate Architecture on Drug Delivery Applications.ACS Biomater Sci Eng. 2024 Nov 11;10(11):6766-6789. doi: 10.1021/acsbiomaterials.4c01105. Epub 2024 Oct 18. ACS Biomater Sci Eng. 2024. PMID: 39423330 Free PMC article. Review.
-
Coacervate Droplets for Synthetic Cells.Small Methods. 2023 Dec;7(12):e2300496. doi: 10.1002/smtd.202300496. Epub 2023 Jul 18. Small Methods. 2023. PMID: 37462244 Review.
Cited by
-
Did the exposure of coacervate droplets to rain make them the first stable protocells?Sci Adv. 2024 Aug 23;10(34):eadn9657. doi: 10.1126/sciadv.adn9657. Epub 2024 Aug 21. Sci Adv. 2024. PMID: 39167649 Free PMC article.
-
Fuel-driven macromolecular coacervation in complex coacervate core micelles.Chem Sci. 2022 Mar 31;13(16):4533-4544. doi: 10.1039/d2sc00805j. eCollection 2022 Apr 20. Chem Sci. 2022. PMID: 35656128 Free PMC article.
-
Enzymatic Reaction Network-Driven Polymerization-Induced Transient Coacervation.Angew Chem Int Ed Engl. 2024 Dec 10;64(11):e202421620. doi: 10.1002/anie.202421620. Online ahead of print. Angew Chem Int Ed Engl. 2024. PMID: 39655501 Free PMC article.
-
ATP:Mg2+ shapes material properties of protein-RNA condensates and their partitioning of clients.Biophys J. 2022 Oct 18;121(20):3962-3974. doi: 10.1016/j.bpj.2022.08.025. Epub 2022 Aug 24. Biophys J. 2022. PMID: 36004782 Free PMC article.
-
Designing negative feedback loops in enzymatic coacervate droplets.Chem Sci. 2023 Apr 19;14(18):4735-4744. doi: 10.1039/d2sc03838b. eCollection 2023 May 10. Chem Sci. 2023. PMID: 37181760 Free PMC article.
References
-
- Bungenberg de Jong H. G.; Kruyt H. R. Coacervation (Partial Miscibility in Colloid Systems). Proc. Acad. Sci. Amsterdam 1929, 32 (1927), 849–856.
-
- Spruijt E.; Westphal A. H.; Borst J. W.; Cohen Stuart M. A.; Van Der Gucht J. Binodal Compositions of Polyelectrolyte Complexes. Macromolecules 2010, 43 (15), 6476–6484. 10.1021/ma101031t. - DOI
-
- Brangwynne C. P.; Tompa P.; Pappu R. V. Polymer Physics of Intracellular Phase Transitions. Nat. Phys. 2015, 11 (11), 899–904. 10.1038/nphys3532. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

