Circuit Integration Initiation of New Hippocampal Neurons in the Adult Brain
- PMID: 31995766
- PMCID: PMC7011119
- DOI: 10.1016/j.celrep.2019.12.084
Circuit Integration Initiation of New Hippocampal Neurons in the Adult Brain
Abstract
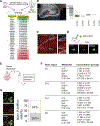
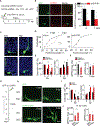
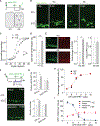
In the adult brain, new dentate granule cells integrate into neural circuits and participate in hippocampal functioning. However, when and how they initiate this integration remain poorly understood. Using retroviral and live-imaging methods, we find that new neurons undergo neurite remodeling for competitive horizontal-to-radial repositioning in the dentate gyrus prior to circuit integration. Gene expression profiling, lipidomics analysis, and molecular interrogation of new neurons during this period reveal a rapid activation of sphingolipid signaling mediated by sphingosine-1-phosphate receptor 1. Genetic manipulation of this G protein-coupled receptor reveals its requirement for successful repositioning of new neurons. This receptor is also activated by hippocampus-engaged behaviors, which enhances repositioning efficiency. These findings reveal that activity-dependent sphingolipid signaling regulates cellular repositioning of new dentate granule cells. The competitive horizontal-to-radial repositioning of new neurons may provide a gating strategy in the adult brain to limit the integration of new neurons into pre-existing circuits.
Keywords: G protein-coupled receptor; adult hippocampal neurogenesis; circuit integration initiation; dentate granule cell; electrophysiological maturation; environmental enrichment; hippocampus behavior-engaged activation; lipidomics; sphingosine-1-phosphate receptor 1.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

References
-
- Altman J, and Das GD (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335. - PubMed
-
- Bielawski J, Szulc ZM, Hannun YA, and Bielawska A (2006). Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39, 82–91. - PubMed
-
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, and Gage FH (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

