Gallbladder wall abnormality in biliary atresia of mouse Sox17+/- neonates and human infants
- PMID: 31996362
- PMCID: PMC7132780
- DOI: 10.1242/dmm.042119
Gallbladder wall abnormality in biliary atresia of mouse Sox17+/- neonates and human infants
Abstract
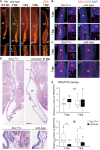
Biliary atresia (BA) is characterized by the inflammation and obstruction of the extrahepatic bile ducts (EHBDs) in newborn infants. SOX17 is a master regulator of fetal EHBD formation. In mouse Sox17+/- BA models, SOX17 reduction causes cell-autonomous epithelial shedding together with the ectopic appearance of SOX9-positive cystic duct-like epithelia in the gallbladder walls, resulting in BA-like symptoms during the perinatal period. However, the similarities with human BA gallbladders are still unclear. In the present study, we conducted phenotypic analysis of Sox17+/- BA neonate mice, in order to compare with the gallbladder wall phenotype of human BA infants. The most characteristic phenotype of the Sox17+/- BA gallbladders is the ectopic appearance of SOX9-positive peribiliary glands (PBGs), so-called pseudopyloric glands (PPGs). Next, we examined SOX17/SOX9 expression profiles of human gallbladders in 13 BA infants. Among them, five BA cases showed a loss or drastic reduction of SOX17-positive signals throughout the whole region of gallbladder epithelia (SOX17-low group). Even in the remaining eight gallbladders (SOX17-high group), the epithelial cells near the decidual sites were frequently reduced in the SOX17-positive signal intensity. Most interestingly, the most characteristic phenotype of human BA gallbladders is the increased density of PBG/PPG-like glands in the gallbladder body, especially near the epithelial decidual site, indicating that PBG/PPG formation is a common phenotype between human BA and mouse Sox17+/- BA gallbladders. These findings provide the first evidence of the potential contribution of SOX17 reduction and PBG/PPG formation to the early pathogenesis of human BA gallbladders.This article has an associated First Person interview with the joint first authors of the paper.
Keywords: Biliary atresia; Cholecystitis; Human; Mouse; PBG; PPG; Peribiliary gland; Pseudopyloric gland; SOX17.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

