Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for metastatic non-small cell lung cancer: retrospective analysis of two single-institution prospective trials
- PMID: 31996395
- PMCID: PMC7057428
- DOI: 10.1136/jitc-2019-000492
Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for metastatic non-small cell lung cancer: retrospective analysis of two single-institution prospective trials
Erratum in
-
Correction: Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for metastatic non-small cell lung cancer: retrospective analysis of two single-institution prospective trials.J Immunother Cancer. 2020 Apr;8(1):e000492corr1. doi: 10.1136/jitc-2019-000492corr1. J Immunother Cancer. 2020. PMID: 32303617 Free PMC article. No abstract available.
Abstract
Background: This study compared response rates and outcomes of combined radiotherapy and immunotherapy (iRT) based on the type of checkpoint inhibitor (anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) vs antiprogrammed death-1 (PD1)) for metastatic non-small cell lung cancer (mNSCLC).
Methods: We retrospectively reviewed two prospective trials of radiation combined with anti-CTLA4 or anti-PD1 for patients with mNSCLC. Patients undergoing non-salvage stereotactic body radiation therapy (SBRT) to lung sites were selected from both trials and grouped by the immunotherapeutic compound received. Endpoints included in-field and out-of-field response rates, and overall response rate (complete or partial response) (all by response evaluation criteria in solid tumors). Progression-free survival (PFS) and overall survival (OS) were estimated with the Kaplan-Meier method.
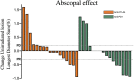
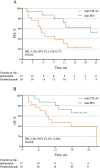
Results: Median follow-up times for the 33 patients (n=17 SBRT+anti-CTLA4, n=16 SBRT+anti-PD1) were 19.6 and 19.9 months. Response rates for out-of-field lesions were similar between anti-PD1 (37%) and anti-CTLA4 (24%) (p=0.054). However, global response rates for all lesions were 24% anti-CTLA4 vs 56% anti-PD1 (p=0.194). The PFS was 76% for anti-CTLA4 vs 94% anti-PD1 at 3 months, 52% vs 87% at 6 months, 31% vs 80% at 12 months, and 23% vs 63% at 18 months (p=0.02). Respective OS values were 76% vs 87% at 6 months, 47% vs 80% at 12 months, and 39% vs 66% at 18 months (p=0.08).
Conclusions: Both anti-CTLA4 and anti-PD1 agents prompt a similar degree of in-field and out-of-field responses after iRT, although the global response rate and PFS were statistically higher in the anti-PD1 cohort. Further dedicated study and biological mechanistic assessment is required.
Trial registration numbers: NCT02239900 and NCT02444741.
Keywords: immunotherapy; radiotherapy.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JW has received grants from Bristol-Myers Squibb, Merck, Varian, and OncoResponse; he also is a cofounder of Healios, MolecularMatch, and OncoResponse (with ownership interest); he is on the scientific advisor board of Mavu, Reflexion Medical and Checkmate Pharmaceuticals, and receives laboratory research support from Varian, Incyte, Calithera, and Checkmate Pharmaceuticals.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
