Toxoplasma gondii Dysregulates Barrier Function and Mechanotransduction Signaling in Human Endothelial Cells
- PMID: 31996420
- PMCID: PMC6992369
- DOI: 10.1128/mSphere.00550-19
Toxoplasma gondii Dysregulates Barrier Function and Mechanotransduction Signaling in Human Endothelial Cells
Abstract
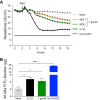
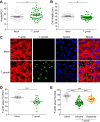
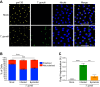
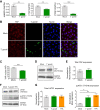
Toxoplasma gondii can infect and replicate in vascular endothelial cells prior to entering host tissues. However, little is known about the molecular interactions at the parasite-endothelial cell interface. We demonstrate that T. gondii infection of primary human umbilical vein endothelial cells (HUVEC) altered cell morphology and dysregulated barrier function, increasing permeability to low-molecular-weight polymers. T. gondii disrupted vascular endothelial cadherin (VE-cadherin) and β-catenin localization to the cell periphery and reduced VE-cadherin protein expression. Notably, T. gondii infection led to reorganization of the host cytoskeleton by reducing filamentous actin (F-actin) stress fiber abundance under static and microfluidic shear stress conditions and by reducing planar cell polarity. RNA sequencing (RNA-Seq) comparing genome-wide transcriptional profiles of infected to uninfected endothelial cells revealed changes in gene expression associated with cell-cell adhesion, extracellular matrix reorganization, and cytokine-mediated signaling. In particular, genes downstream of Hippo signaling and the biomechanical sensor and transcriptional coactivator Yes-associated protein (YAP) were downregulated in infected endothelial cells. Interestingly, T. gondii infection activated Hippo signaling by increasing phosphorylation of LATS1, leading to cytoplasmic retention of YAP, and reducing YAP target gene expression. These findings suggest that T. gondii infection triggers Hippo signaling and YAP nuclear export, leading to an altered transcriptional profile of infected endothelial cells.IMPORTANCE Toxoplasma gondii is a foodborne parasite that infects virtually all warm-blooded animals and can cause severe disease in individuals with compromised or weakened immune systems. During dissemination in its infected hosts, T. gondii breaches endothelial barriers to enter tissues and establish the chronic infections underlying the most severe manifestations of toxoplasmosis. The research presented here examines how T. gondii infection of primary human endothelial cells induces changes in cell morphology, barrier function, gene expression, and mechanotransduction signaling under static conditions and under the physiological conditions of shear stress found in the bloodstream. Understanding the molecular interactions occurring at the interface between endothelial cells and T. gondii may provide insights into processes linked to parasite dissemination and pathogenesis.
Keywords: Hippo signaling; Toxoplasma gondii; VE-cadherin; actin; endothelial cell; mechanotransduction.
Copyright © 2020 Franklin-Murray et al.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

