Effect of HIV Envelope Vaccination on the Subsequent Antibody Response to HIV Infection
- PMID: 31996422
- PMCID: PMC6992371
- DOI: 10.1128/mSphere.00738-19
Effect of HIV Envelope Vaccination on the Subsequent Antibody Response to HIV Infection
Abstract
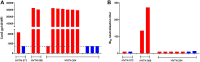
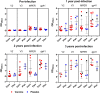
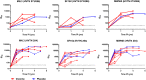
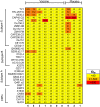
Analysis of breakthrough HIV-1 infections could elucidate whether prior vaccination primes relevant immune responses. Here, we measured HIV-specific antibody responses in 14 South African volunteers who acquired HIV infection after participating in phase 1/2 trials of envelope-containing immunogens. Serum samples were collected annually following HIV-1 infection from participants in trials HVTN 073 (subtype C, DNA/MVA, phase 1 trial, n = 1), HVTN 086 (subtype C, DNA/MVA/gp140 protein, phase 1 trial, n = 2), and HVTN 204 (multisubtype, DNA/adenovirus serotype 5 [Ad5], phase 2 trial, n = 7) and 4 placebo recipients. Binding and neutralizing antibody responses to Env proteins and peptides were determined pre- and post-HIV infection using an enzyme-linked immunosorbent assay and the TZM-bl cell neutralization assay, respectively. HIV-infected South African individuals served as unvaccinated controls. Binding antibodies to gp41, V3, V2, the membrane-proximal external region (MPER), and the CD4 binding site were detected from the first year of HIV-1 subtype C infection, and the levels were similar in vaccinated and placebo recipients. Neutralizing antibody responses against tier 1A viruses were detected in all participants, with the highest titers being to a subtype C virus, MW965.26. No responses were observed just prior to infection, indicating that vaccine-primed HIV-specific antibodies had waned. Sporadic neutralization activity against tier 2 isolates was observed after 2 to 3 years of HIV infection, but these responses were similar in the vaccinated and placebo groups as well as the unvaccinated controls. Our data suggest that prior vaccination with these immunogens did not alter the antibody responses to HIV-1 infection, nor did it accelerate the development of HIV neutralization breadth.IMPORTANCE There is a wealth of information on HIV-specific vaccine-induced immune responses among HIV-uninfected participants; however, data on immune responses among participants who acquire HIV after vaccination are limited. Here we show that HIV-specific binding antibody responses in individuals with breakthrough HIV infections were not affected by prior vaccination with HIV envelope-containing immunogens. We also found that these vectored vaccines did not prime tier 2 virus-neutralizing antibody responses, which are thought to be required for prevention against HIV acquisition, or accelerate the development of neutralization breadth. Although this study is limited, such studies can provide insights into whether vaccine-elicited antibody responses are boosted by HIV infection to acquire broader neutralizing activity, which may help to identify antigens relevant to the design of more effective vaccines.
Keywords: DNA/MVA vaccines; HIV breakthrough infections; HIV vaccines; HIV-1 envelope; HIV-specific binding antibodies; binding antibody epitopes; broadly neutralizing antibodies; rAd5.
Copyright © 2020 Ditse et al.
Figures

References
-
- UNAIDS. 2018. Global HIV and AIDS statistics—2018 fact sheet. UNAIDS, Geneva, Switzerland.
-
- Gilbert P, Wang M, Wrin T, Petropoulos C, Gurwith M, Sinangil F, D'Souza P, Rodriguez-Chavez IR, DeCamp A, Giganti M, Berman PW, Self SG, Montefiori DC. 2010. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV‐1 gp120 vaccine. J Infect Dis 202:595–605. doi: 10.1086/654816. - DOI - PMC - PubMed
-
- Mascola JR, Snyder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, Clements ML, Dolin R, Graham BS, Gorse GJ, Keefer MC, McElrath MJ, Walker MC, Wagner KF, McNeil JG, McCutchan FE, Burke DS. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis 173:340–348. doi: 10.1093/infdis/173.2.340. - DOI - PubMed
-
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K, Bangkok Vaccine Evaluation Group. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 194:1661–1671. doi: 10.1086/508748. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

