Characterization of Mollivirus kamchatka, the First Modern Representative of the Proposed Molliviridae Family of Giant Viruses
- PMID: 31996429
- PMCID: PMC7108836
- DOI: 10.1128/JVI.01997-19
Characterization of Mollivirus kamchatka, the First Modern Representative of the Proposed Molliviridae Family of Giant Viruses
Abstract
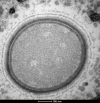
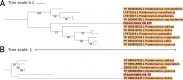
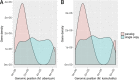
Microbes trapped in permanently frozen paleosoils (permafrost) are the focus of increasing research in the context of global warming. Our previous investigations led to the discovery and reactivation of two Acanthamoeba-infecting giant viruses, Mollivirus sibericum and Pithovirus sibericum, from a 30,000-year old permafrost layer. While several modern pithovirus strains have since been isolated, no contemporary mollivirus relative was found. We now describe Mollivirus kamchatka, a close relative to M. sibericum, isolated from surface soil sampled on the bank of the Kronotsky River in Kamchatka, Russian Federation. This discovery confirms that molliviruses have not gone extinct and are at least present in a distant subarctic continental location. This modern isolate exhibits a nucleocytoplasmic replication cycle identical to that of M. sibericum Its spherical particle (0.6 μm in diameter) encloses a 648-kb GC-rich double-stranded DNA genome coding for 480 proteins, of which 61% are unique to these two molliviruses. The 461 homologous proteins are highly conserved (92% identical residues, on average), despite the presumed stasis of M. sibericum for the last 30,000 years. Selection pressure analyses show that most of these proteins contribute to virus fitness. The comparison of these first two molliviruses clarify their evolutionary relationship with the pandoraviruses, supporting their provisional classification in a distinct family, the Molliviridae, pending the eventual discovery of intermediary missing links better demonstrating their common ancestry.IMPORTANCE Virology has long been viewed through the prism of human, cattle, or plant diseases, leading to a largely incomplete picture of the viral world. The serendipitous discovery of the first giant virus visible under a light microscope (i.e., >0.3 μm in diameter), mimivirus, opened a new era of environmental virology, now incorporating protozoan-infecting viruses. Planet-wide isolation studies and metagenome analyses have shown the presence of giant viruses in most terrestrial and aquatic environments, including upper Pleistocene frozen soils. Those systematic surveys have led authors to propose several new distinct families, including the Mimiviridae, Marseilleviridae, Faustoviridae, Pandoraviridae, and Pithoviridae We now propose to introduce one additional family, the Molliviridae, following the description of M. kamchatka, the first modern relative of M. sibericum, previously isolated from 30,000-year-old arctic permafrost.
Keywords: Acanthamoeba; Arctic; Kamchatka; NCLDV; comparative genomics; paleovirology.
Copyright © 2020 American Society for Microbiology.
Figures







References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

