Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia
- PMID: 31996455
- PMCID: PMC7096150
- DOI: 10.1523/JNEUROSCI.2374-19.2020
Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia
Abstract
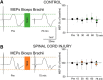
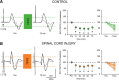
An increasing number of studies supports the view that transcutaneous electrical stimulation of the spinal cord (TESS) promotes functional recovery in humans with spinal cord injury (SCI). However, the neural mechanisms contributing to these effects remain poorly understood. Here we examined motor-evoked potentials in arm muscles elicited by cortical and subcortical stimulation of corticospinal axons before and after 20 min of TESS (30 Hz pulses with a 5 kHz carrier frequency) and sham-TESS applied between C5 and C6 spinous processes in males and females with and without chronic incomplete cervical SCI. The amplitude of subcortical, but not cortical, motor-evoked potentials increased in proximal and distal arm muscles for 75 min after TESS, but not sham-TESS, in control subjects and SCI participants, suggesting a subcortical origin for these effects. Intracortical inhibition, elicited by paired stimuli, increased after TESS in both groups. When TESS was applied without the 5 kHz carrier frequency both subcortical and cortical motor-evoked potentials were facilitated without changing intracortical inhibition, suggesting that the 5 kHz carrier frequency contributed to the cortical inhibitory effects. Hand and arm function improved largely when TESS was used with, compared with without, the 5 kHz carrier frequency. These novel observations demonstrate that TESS influences cortical and spinal networks, having an excitatory effect at the spinal level and an inhibitory effect at the cortical level. We hypothesized that these parallel effects contribute to further the recovery of limb function following SCI.SIGNIFICANCE STATEMENT Accumulating evidence supports the view that transcutaneous electrical stimulation of the spinal cord (TESS) promotes recovery of function in humans with spinal cord injury (SCI). Here, we show that a single session of TESS over the cervical spinal cord in individuals with incomplete chronic cervical SCI influenced in parallel the excitability cortical and spinal networks, having an excitatory effect at the spinal level and an inhibitory effect at the cortical level. Importantly, these parallel physiological effects had an impact on the magnitude of improvements in voluntary motor output.
Keywords: corticospinal; intracortical inhibition; neurophysiology; neuroplasticity; spinal cord injury; spinal networks.
Copyright © 2020 the authors.
Figures





References
-
- Alam M, Garcia-Alias G, Jin B, Keyes J, Zhong H, Roy RR, Gerasimenko Y, Lu DC, Edgerton VR (2017) Electrical neuromodulation of the cervical spinal cord facilitates forelimb skilled function recovery in spinal cord injured rats. Exp Neurol 291:141–150. 10.1016/j.expneurol.2017.02.006 - DOI - PMC - PubMed
-
- Berardelli A, Inghilleri M, Cruccu G, Manfredi M (1991) Corticospinal potentials after electrical and magnetic stimulation in man. Electroencephalogr Clin Neurophysiol Suppl 43:147–154. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
