mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis
- PMID: 31996678
- PMCID: PMC6989638
- DOI: 10.1038/s41467-020-14430-w
mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis
Erratum in
-
Author Correction: mTORC2-AKT signaling to ATP-citrate lyase drives brown adipogenesis and de novo lipogenesis.Nat Commun. 2020 Sep 8;11(1):4585. doi: 10.1038/s41467-020-18510-9. Nat Commun. 2020. PMID: 32901020 Free PMC article.
Abstract
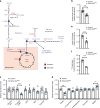
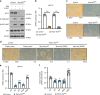
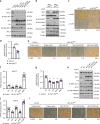
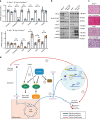
mTORC2 phosphorylates AKT in a hydrophobic motif site that is a biomarker of insulin sensitivity. In brown adipocytes, mTORC2 regulates glucose and lipid metabolism, however the mechanism has been unclear because downstream AKT signaling appears unaffected by mTORC2 loss. Here, by applying immunoblotting, targeted phosphoproteomics and metabolite profiling, we identify ATP-citrate lyase (ACLY) as a distinctly mTORC2-sensitive AKT substrate in brown preadipocytes. mTORC2 appears dispensable for most other AKT actions examined, indicating a previously unappreciated selectivity in mTORC2-AKT signaling. Rescue experiments suggest brown preadipocytes require the mTORC2/AKT/ACLY pathway to induce PPAR-gamma and establish the epigenetic landscape during differentiation. Evidence in mature brown adipocytes also suggests mTORC2 acts through ACLY to increase carbohydrate response element binding protein (ChREBP) activity, histone acetylation, and gluco-lipogenic gene expression. Substrate utilization studies additionally implicate mTORC2 in promoting acetyl-CoA synthesis from acetate through acetyl-CoA synthetase 2 (ACSS2). These data suggest that a principal mTORC2 action is controlling nuclear-cytoplasmic acetyl-CoA synthesis.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

