Activation of pH-Sensing Receptor OGR1 (GPR68) Induces ER Stress Via the IRE1α/JNK Pathway in an Intestinal Epithelial Cell Model
- PMID: 31996710
- PMCID: PMC6989664
- DOI: 10.1038/s41598-020-57657-9
Activation of pH-Sensing Receptor OGR1 (GPR68) Induces ER Stress Via the IRE1α/JNK Pathway in an Intestinal Epithelial Cell Model
Abstract
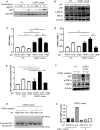
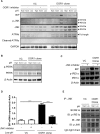
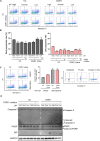
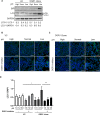
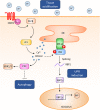
Proton-sensing ovarian cancer G-protein coupled receptor (OGR1) plays an important role in pH homeostasis. Acidosis occurs at sites of intestinal inflammation and can induce endoplasmic reticulum (ER) stress and the unfolded protein response (UPR), an evolutionary mechanism that enables cells to cope with stressful conditions. ER stress activates autophagy, and both play important roles in gut homeostasis and contribute to the pathogenesis of inflammatory bowel disease (IBD). Using a human intestinal epithelial cell model, we investigated whether our previously observed protective effects of OGR1 deficiency in experimental colitis are associated with a differential regulation of ER stress, the UPR and autophagy. Caco-2 cells stably overexpressing OGR1 were subjected to an acidic pH shift. pH-dependent OGR1-mediated signalling led to a significant upregulation in the ER stress markers, binding immunoglobulin protein (BiP) and phospho-inositol required 1α (IRE1α), which was reversed by a novel OGR1 inhibitor and a c-Jun N-terminal kinase (JNK) inhibitor. Proton-activated OGR1-mediated signalling failed to induce apoptosis, but triggered accumulation of total microtubule-associated protein 1 A/1B-light chain 3, suggesting blockage of late stage autophagy. Our results show novel functions for OGR1 in the regulation of ER stress through the IRE1α-JNK signalling pathway, as well as blockage of autophagosomal degradation. OGR1 inhibition might represent a novel therapeutic approach in IBD.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
IRE1α-JNK pathway-mediated autophagy promotes cell survival in response to endoplasmic reticulum stress during the initial phase of hepatic steatosis.Life Sci. 2021 Jan 1;264:118668. doi: 10.1016/j.lfs.2020.118668. Epub 2020 Oct 26. Life Sci. 2021. PMID: 33121987
-
The pH-sensing receptor OGR1 improves barrier function of epithelial cells and inhibits migration in an acidic environment.Am J Physiol Gastrointest Liver Physiol. 2015 Sep 15;309(6):G475-90. doi: 10.1152/ajpgi.00408.2014. Epub 2015 Jul 23. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26206859
-
IRE1α dissociates with BiP and inhibits ER stress-mediated apoptosis in cartilage development.Cell Signal. 2013 Nov;25(11):2136-46. doi: 10.1016/j.cellsig.2013.06.011. Epub 2013 Jun 29. Cell Signal. 2013. PMID: 23816533
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
-
The multiple roles of the unfolded protein response regulator IRE1α in cancer.Mol Carcinog. 2019 Sep;58(9):1623-1630. doi: 10.1002/mc.23031. Epub 2019 Apr 30. Mol Carcinog. 2019. PMID: 31041814 Free PMC article. Review.
Cited by
-
Mechanosensitive Regulation of Fibrosis.Cells. 2021 Apr 23;10(5):994. doi: 10.3390/cells10050994. Cells. 2021. PMID: 33922651 Free PMC article. Review.
-
Role of pH-sensing receptors in colitis.Pflugers Arch. 2024 Apr;476(4):611-622. doi: 10.1007/s00424-024-02943-y. Epub 2024 Mar 22. Pflugers Arch. 2024. PMID: 38514581 Free PMC article. Review.
-
Proton-Sensing GPCRs in Health and Disease.Cells. 2021 Aug 10;10(8):2050. doi: 10.3390/cells10082050. Cells. 2021. PMID: 34440817 Free PMC article. Review.
-
Identification of an endoplasmic reticulum stress-related prognostic risk model with excellent prognostic and clinical value in oral squamous cell carcinoma.Aging (Albany NY). 2023 Aug 25;15(19):10010-10030. doi: 10.18632/aging.204983. Epub 2023 Aug 25. Aging (Albany NY). 2023. PMID: 37647077 Free PMC article.
-
Nobiletin in Cancer Therapy: How This Plant Derived-Natural Compound Targets Various Oncogene and Onco-Suppressor Pathways.Biomedicines. 2020 May 5;8(5):110. doi: 10.3390/biomedicines8050110. Biomedicines. 2020. PMID: 32380783 Free PMC article. Review.
References
-
- Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69:522–530. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

