Cell stress in cortical organoids impairs molecular subtype specification
- PMID: 31996853
- PMCID: PMC7433012
- DOI: 10.1038/s41586-020-1962-0
Cell stress in cortical organoids impairs molecular subtype specification
Abstract
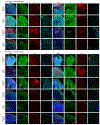
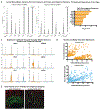
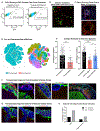
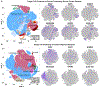
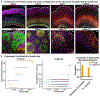
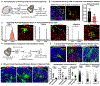
Cortical organoids are self-organizing three-dimensional cultures that model features of the developing human cerebral cortex1,2. However, the fidelity of organoid models remains unclear3-5. Here we analyse the transcriptomes of individual primary human cortical cells from different developmental periods and cortical areas. We find that cortical development is characterized by progenitor maturation trajectories, the emergence of diverse cell subtypes and areal specification of newborn neurons. By contrast, organoids contain broad cell classes, but do not recapitulate distinct cellular subtype identities and appropriate progenitor maturation. Although the molecular signatures of cortical areas emerge in organoid neurons, they are not spatially segregated. Organoids also ectopically activate cellular stress pathways, which impairs cell-type specification. However, organoid stress and subtype defects are alleviated by transplantation into the mouse cortex. Together, these datasets and analytical tools provide a framework for evaluating and improving the accuracy of cortical organoids as models of human brain development.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures
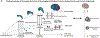












Comment in
-
Imposter syndrome?Nat Rev Neurosci. 2020 Apr;21(4):180-181. doi: 10.1038/s41583-020-0280-8. Nat Rev Neurosci. 2020. PMID: 32080403 No abstract available.
-
Benchmarking pluripotent stem cell-derived organoid models.Exp Neurol. 2020 Aug;330:113333. doi: 10.1016/j.expneurol.2020.113333. Epub 2020 Apr 27. Exp Neurol. 2020. PMID: 32353463
References
-
- Kadoshima T et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proceedings of the National Academy of Sciences of the United States of America 110, 20284–20289, doi: 10.1073/pnas.1315710110 (2013). - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

