Synergistic defects in pre-rRNA processing from mutations in the U3-specific protein Rrp9 and U3 snoRNA
- PMID: 31996908
- PMCID: PMC7144924
- DOI: 10.1093/nar/gkaa066
Synergistic defects in pre-rRNA processing from mutations in the U3-specific protein Rrp9 and U3 snoRNA
Abstract
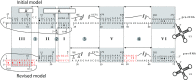
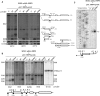
U3 snoRNA and the associated Rrp9/U3-55K protein are essential for 18S rRNA production by the SSU-processome complex. U3 and Rrp9 are required for early pre-rRNA cleavages at sites A0, A1 and A2, but the mechanism remains unclear. Substitution of Arg 289 in Rrp9 to Ala (R289A) specifically reduced cleavage at sites A1 and A2. Surprisingly, R289 is located on the surface of the Rrp9 β-propeller structure opposite to U3 snoRNA. To understand this, we first characterized the protein-protein interaction network of Rrp9 within the SSU-processome. This identified a direct interaction between the Rrp9 β-propeller domain and Rrp36, the strength of which was reduced by the R289A substitution, implicating this interaction in the observed processing phenotype. The Rrp9 R289A mutation also showed strong synergistic negative interactions with mutations in U3 that destabilize the U3/pre-rRNA base-pair interactions or reduce the length of their linking segments. We propose that the Rrp9 β-propeller and U3/pre-rRNA binding cooperate in the structure or stability of the SSU-processome. Additionally, our analysis of U3 variants gave insights into the function of individual segments of the 5'-terminal 72-nt sequence of U3. We interpret these data in the light of recently reported SSU-processome structures.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures








Similar articles
-
Structural and functional analysis of the U3 snoRNA binding protein Rrp9.RNA. 2013 May;19(5):701-11. doi: 10.1261/rna.037580.112. Epub 2013 Mar 18. RNA. 2013. PMID: 23509373 Free PMC article.
-
A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis.Nature. 2002 Jun 27;417(6892):967-70. doi: 10.1038/nature00769. Epub 2002 Jun 9. Nature. 2002. PMID: 12068309 Free PMC article.
-
Trypanosoma brucei 5'ETS A'-cleavage is directed by 3'-adjacent sequences, but not two U3 snoRNA-binding elements, which are all required for subsequent pre-small subunit rRNA processing events.J Mol Biol. 2001 Nov 2;313(4):733-49. doi: 10.1006/jmbi.2001.5078. J Mol Biol. 2001. PMID: 11697900
-
Mpp10p, a new protein component of the U3 snoRNP required for processing of 18S rRNA precursors.Nucleic Acids Symp Ser. 1997;(36):64-7. Nucleic Acids Symp Ser. 1997. PMID: 9478208 Review.
-
snR30/U17 Small Nucleolar Ribonucleoprotein: A Critical Player during Ribosome Biogenesis.Cells. 2020 Sep 29;9(10):2195. doi: 10.3390/cells9102195. Cells. 2020. PMID: 33003357 Free PMC article. Review.
Cited by
-
Analysis and Validation of Autophagy-Related Gene Biomarkers and Immune Cell Infiltration Characteristic in Bronchopulmonary Dysplasia by Integrating Bioinformatics and Machine Learning.J Inflamm Res. 2025 Jan 13;18:549-563. doi: 10.2147/JIR.S495132. eCollection 2025. J Inflamm Res. 2025. PMID: 39839185 Free PMC article.
-
Regulatory Non-Coding RNAs: An Overview.Methods Mol Biol. 2021;2300:3-9. doi: 10.1007/978-1-0716-1386-3_1. Methods Mol Biol. 2021. PMID: 33792866 Review.
-
Advancements in colorectal cancer research: Unveiling the cellular and molecular mechanisms of neddylation (Review).Int J Oncol. 2024 Apr;64(4):39. doi: 10.3892/ijo.2024.5627. Epub 2024 Feb 23. Int J Oncol. 2024. PMID: 38391033 Free PMC article. Review.
-
RRP9 promotes prostate cancer metastasis and epithelial-mesenchymal transition through activation of the AKT/GSK3β/β-Catenin signaling pathway.Discov Oncol. 2025 Jun 17;16(1):1129. doi: 10.1007/s12672-025-02833-4. Discov Oncol. 2025. PMID: 40526312 Free PMC article.
-
Multiomics Integrated Analysis Identifies SLC24A2 as a Potential Link between Type 2 Diabetes and Cancer.J Diabetes Res. 2022 May 13;2022:4629419. doi: 10.1155/2022/4629419. eCollection 2022. J Diabetes Res. 2022. PMID: 35601016 Free PMC article.
References
-
- Brand R.C., Planta R.J.. The molecular weights of yeast ribosomal precursor RNAs. Mol. Biol. Rep. 1975; 2:321–325. - PubMed
-
- Granneman S., Baserga S.J.. Ribosome biogenesis: of knobs and RNA processing. Exp. Cell Res. 2004; 296:43–50. - PubMed
-
- Bachellerie J.P., Cavaillé J.. Guiding ribose methylation of rRNA. Trends Biochem. Sci. 1997; 22:257–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

