Selected microRNAs Increase Synaptic Resilience to the Damaging Binding of the Alzheimer's Disease Amyloid Beta Oligomers
- PMID: 31997075
- PMCID: PMC7170988
- DOI: 10.1007/s12035-020-01868-8
Selected microRNAs Increase Synaptic Resilience to the Damaging Binding of the Alzheimer's Disease Amyloid Beta Oligomers
Abstract
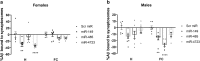
Alzheimer's disease (AD) is marked by synaptic loss (at early stages) and neuronal death (at late stages). Amyloid beta (Aβ) and tau oligomers can target and disrupt synapses thus driving cognitive decay. Non-demented individuals with Alzheimer's neuropathology (NDAN) are capable of withstanding Aβ and tau toxicity, thus remaining cognitively intact despite presence of AD neuropathology. Understanding the involved mechanism(s) would lead to development of novel effective therapeutic strategies aimed at promoting synaptic resilience to amyloid toxicity. NDAN have a unique hippocampal post-synaptic proteome when compared with AD and control individuals. Potential upstream modulators of such unique proteomic profile are miRNA-485, miRNA-4723 and miRNA-149, which we found differentially expressed in AD and NDAN vs. control. We thus hypothesized that these miRNAs play an important role in promoting either synaptic resistance or sensitization to Aβ oligomer binding. Using an in vivo mouse model, we found that administration of these miRNAs affected key synaptic genes and significantly decreased Aβ binding to the synapses. Our findings suggest that miRNA regulation and homeostasis are crucial for Aβ interaction with synaptic terminals and support that a unique miRNA regulation could be driving synaptic resistance to Aβ toxicity in NDAN, thus contributing to their preserved cognitive abilities.
Keywords: Alzheimer’s disease; Non-demented with Alzheimer’s neuropathology; Synaptic resilience; microRNA.
Figures


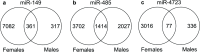

References
-
- Alzheimer’s and dementia (2018) Alzheimer’s facts and figures report Alzheimer’s Association. https://www.alz.org/alzheimers-dementia/facts-figures. Accessed 14 Sep 2018
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

