Interactions of 17β-Hydroxysteroid Dehydrogenase Type 10 and Cyclophilin D in Alzheimer's Disease
- PMID: 31997103
- PMCID: PMC7078148
- DOI: 10.1007/s11064-020-02970-y
Interactions of 17β-Hydroxysteroid Dehydrogenase Type 10 and Cyclophilin D in Alzheimer's Disease
Abstract
The nucleus-encoded 17β-hydroxysteroid dehydrogenase type 10 (17β-HSD10) regulates cyclophilin D (cypD) in the mitochondrial matrix. CypD regulates opening of mitochondrial permeability transition pores. Both mechanisms may be affected by amyloid β peptides accumulated in mitochondria in Alzheimer's disease (AD). In order to clarify changes occurring in brain mitochondria, we evaluated interactions of both mitochondrial proteins in vitro (by surface plasmon resonance biosensor) and detected levels of various complexes of 17β-HSD10 formed in vivo (by sandwich ELISA) in brain mitochondria isolated from the transgenic animal model of AD (homozygous McGill-R-Thy1-APP rats) and in cerebrospinal fluid samples of AD patients. By surface plasmon resonance biosensor, we observed the interaction of 17β-HSD10 and cypD in a direct real-time manner and determined, for the first time, the kinetic parameters of the interaction (ka 2.0 × 105 M1s-1, kd 5.8 × 104 s-1, and KD 3.5 × 10-10 M). In McGill-R-Thy1-APP rats compared to controls, levels of 17β-HSD10-cypD complexes were decreased and those of total amyloid β increased. Moreover, the levels of 17β-HSD10-cypD complexes were decreased in cerebrospinal fluid of individuals with AD (in mild cognitive impairment as well as dementia stages) or with Frontotemporal lobar degeneration (FTLD) compared to cognitively normal controls (the sensitivity of the complexes to AD dementia was 92.9%, that to FTLD 73.8%, the specificity to AD dementia equaled 91.7% in a comparison with the controls but only 26.2% with FTLD). Our results demonstrate the weakened ability of 17β-HSD10 to regulate cypD in the mitochondrial matrix probably via direct effects of amyloid β. Levels of 17β-HSD10-cypD complexes in cerebrospinal fluid seem to be the very sensitive indicator of mitochondrial dysfunction observed in neurodegeneration but unfortunately not specific to AD pathology. We do not recommend it as the new biomarker of AD.
Keywords: Alzheimer's disease; Amyloid β; Cerebrospinal fluid; Frontotemporal lobar degeneration; Mitochondrial matrix proteins; Transgenic rat model.
Figures


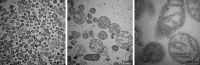

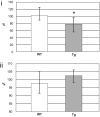
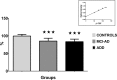
References
-
- Rauschenberger K, Schöler K, Sass JO, Sauer S, Djuric Z, Rumig C, Wolf NI, Okun JG, Kolker S, Schwarz H, Fisher C, Grziwa B, Runz H, Numann A, Shafqat N, Kavanagh KL, Hammerling G, Wanders RJA, Shield JPH, Wendel U, Stern D, Nawroth P, Hoffmann GF, Bartram CR, Arnold B, Bierhaus A, Oppermann U, Steinbeisser H, Zschocke J. A non-enzymatic function of 17β-hydroxysteroid dehydrogenase type 10 is required for mitochondrial integrity and cell survival. EMBO Mol Med. 2010;2:51–62. doi: 10.1002/emmm.200900055. - DOI - PMC - PubMed
-
- Bertolin G, Jacoupy M, Traver S, Ferrendo-Miguel R, Saint Georges T, Grenier K, Ardila-Osorio H, Muriel MP, Takahashi H, Lees AJ, Gautier C, Guedin D, Coge F, Fon EA, Brice A, Corti O. Parkin maintains mitochondrial levels of the protective Parkinson's disease-related enzyme 17-β hydroxysteroid dehydrogenase type 10. Cell Death Differ. 2015;22:1563–1576. doi: 10.1038/cdd.2014.224. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

