Induction of tryptophan hydroxylase in the liver of s.c. tumor model of prostate cancer
- PMID: 31997472
- PMCID: PMC7156786
- DOI: 10.1111/cas.14333
Induction of tryptophan hydroxylase in the liver of s.c. tumor model of prostate cancer
Abstract
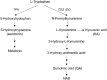
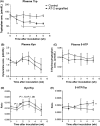
Enhanced degradation of tryptophan (Trp) and thus decreased plasma Trp levels are common in several types of cancers. Although it is well known that Trp catabolism is induced in the tumor microenvironment by the enzymes expressed in cancer cells, immune cells, or both, few studies have examined systemic Trp catabolism in cancer pathophysiology. The present study aimed to evaluate Trp catabolism in both tumor and peripheral tissues using tumor-engrafted Copenhagen rats that were s.c. inoculated with AT-2 rat prostate cancer cells negative for expression of Trp catabolic enzymes. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) metabolomics showed significantly decreased plasma Trp levels in AT-2 engrafted rats, accompanied by increased kynurenine/Trp ratios in spleen and thymus and serotonin levels in liver and thymus. Quantitative PCR and enzymatic activity assays showed indoleamine-2, 3-dioxygenase, an inducible enzyme that catalyzes Trp to kynurenine, was increased in tumor tissues, whereas tryptophan-2,3-dioxygenase, a major Trp catabolic enzyme that regulates systemic level of Trp, tended to be increased in the liver of AT-2 engrafted rats. Furthermore, tryptophan hydroxylase-1 (TPH1), an enzyme that catalyzes the reaction of Trp to serotonin, was significantly increased in liver and spleen of AT-2 engrafted rats. Further histochemical analysis revealed that the induction of TPH1 in the liver could be attributed to infiltration of mast cells. A similar phenomenon was observed with nonneoplastic liver samples from colorectal cancer patients. These results suggested that Trp catabolism toward serotonin synthesis might be induced in peripheral remote tissues in cancer, which could have a pathophysiological effect on cancer.
Keywords: liver; mast cell; serotonin; tryptophan; tryptophan hydroxylase.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
AH, RN, and SU are employees of Ajinomoto Co., Inc. YN and YM declare that they have no conflict of interest.
Figures




References
-
- van der Goot AT, Nollen EA. Tryptophan metabolism: entering the field of aging and age‐related pathologies. Trends Mol Med. 2013;19:336‐344. - PubMed
-
- Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017;357. - PubMed
-
- Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72:5435‐5440. - PubMed
-
- Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3‐dioxygenase. Nat Med. 2003;9:1269‐1274. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

