Phospho-inositide-dependent kinase 1 regulates signal dependent translation in megakaryocytes and platelets
- PMID: 31997536
- PMCID: PMC7192796
- DOI: 10.1111/jth.14748
Phospho-inositide-dependent kinase 1 regulates signal dependent translation in megakaryocytes and platelets
Abstract
Background: Regulated protein synthesis is essential for megakaryocyte (MK) and platelet functions, including platelet production and activation. PDK1 (phosphoinositide-dependent kinase 1) regulates platelet functional responses and has been associated with circulating platelet counts. Whether PDK1 also directly regulates protein synthetic responses in MKs and platelets, and platelet production by MKs, remains unknown.
Objective: To determine if PDK1 regulates protein synthesis in MKs and platelets.
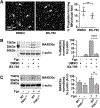
Methods: Pharmacologic PDK1 inhibitors (BX-795) and mice where PDK1 was selectively ablated in MKs and platelets (PDK1-/- ) were used. PDK1 signaling in MKs and platelets (human and murine) were assessed by immunoblots. Activation-dependent translation initiation and protein synthesis in MKs and platelets was assessed by probing for dissociation of eIF4E from 4EBP1, and using m7-GTP pulldowns and S35 methionine incorporation assays. Proplatelet formation by MKs, synthesis of Bcl-3 and MARCKs protein, and clot retraction were employed for functional assays.
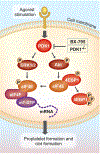
Results: Inhibiting or ablating PDK1 in MKs and platelets abolished the phosphorylation of 4EBP1 and eIF4E by preventing activation of the PI3K and MAPK pathways. Inhibiting PDK1 also prevented dissociation of eIF4E from 4EBP1, decreased binding of eIF4E to m7GTP (required for translation initiation), and significantly reduced de novo protein synthesis. Inhibiting PDK1 reduced proplatelet formation by human MKs and blocked MARCKs protein synthesis. In both human and murine platelets, PDK1 controlled Bcl-3 synthesis. Inhibition of PDK1 led to complete failure of clot retraction in vitro.
Conclusions: PDK1 is a previously unidentified translational regulator in MKs and platelets, controlling protein synthetic responses, proplatelet formation, and clot retraction.
Keywords: platelet activation; platelets; protein translation; signal transduction; thrombosis.
© 2020 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Disclosure of Conflict of Interests
The authors state that they have no relevant conflict of interest.
Figures



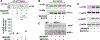

Similar articles
-
Pivotal role of PDK1 in megakaryocyte cytoskeletal dynamics and polarization during platelet biogenesis.Blood. 2019 Nov 21;134(21):1847-1858. doi: 10.1182/blood.2019000185. Blood. 2019. PMID: 31578203
-
Synthesis and dephosphorylation of MARCKS in the late stages of megakaryocyte maturation drive proplatelet formation.Blood. 2016 Mar 17;127(11):1468-80. doi: 10.1182/blood-2015-08-663146. Epub 2016 Jan 7. Blood. 2016. PMID: 26744461 Free PMC article.
-
PDK1 governs thromboxane generation and thrombosis in platelets by regulating activation of Raf1 in the MAPK pathway.J Thromb Haemost. 2018 Jun;16(6):1211-1225. doi: 10.1111/jth.14005. Epub 2018 May 8. J Thromb Haemost. 2018. PMID: 29575487 Free PMC article.
-
The heterogeneity of megakaryocytes and platelets and implications for ex vivo platelet generation.Stem Cells Transl Med. 2021 Dec;10(12):1614-1620. doi: 10.1002/sctm.21-0264. Epub 2021 Sep 18. Stem Cells Transl Med. 2021. PMID: 34536061 Free PMC article. Review.
-
In vivo platelet production from mature megakaryocytes: does platelet release occur via proplatelets?Int J Hematol. 2005 Apr;81(3):208-19. doi: 10.1532/IJH97.04177. Int J Hematol. 2005. PMID: 15814332 Review.
Cited by
-
A New Role of NAP1L1 in Megakaryocytes and Human Platelets.Int J Mol Sci. 2022 Nov 24;23(23):14694. doi: 10.3390/ijms232314694. Int J Mol Sci. 2022. PMID: 36499021 Free PMC article.
-
Genetically engineered transfusable platelets using mRNA lipid nanoparticles.Sci Adv. 2023 Dec;9(48):eadi0508. doi: 10.1126/sciadv.adi0508. Epub 2023 Dec 1. Sci Adv. 2023. PMID: 38039367 Free PMC article.
-
Human platelets display dysregulated sepsis-associated autophagy, induced by altered LC3 protein-protein interaction of the Vici-protein EPG5.Autophagy. 2022 Jul;18(7):1534-1550. doi: 10.1080/15548627.2021.1990669. Epub 2021 Nov 18. Autophagy. 2022. PMID: 34689707 Free PMC article.
-
MAPK-interacting kinase 1 regulates platelet production, activation, and thrombosis.Blood. 2022 Dec 8;140(23):2477-2489. doi: 10.1182/blood.2022015568. Blood. 2022. PMID: 35930749 Free PMC article.
-
Role of Rho-GTPases in megakaryopoiesis.Small GTPases. 2021 Sep-Nov;12(5-6):399-415. doi: 10.1080/21541248.2021.1885134. Epub 2021 Feb 11. Small GTPases. 2021. PMID: 33570449 Free PMC article. Review.
References
-
- Machlus KR, Wu SK, Stumpo DJ, Soussou TS, Paul DS, Campbell RA, Kalwa H, Michel T, Bergmeier W, Weyrich AS, Blackshear PJ, Hartwig JH, Italiano JE Jr, Synthesis and dephosphorylation of MARCKS in the late stages of megakaryocyte maturation drive proplatelet formation. Blood. 2016; 127: 1468–80. 10.1182/blood-2015-08-663146. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

