A longitudinal analysis of the completeness of maternal HIV testing, including repeat testing in Cape Town, South Africa
- PMID: 31997583
- PMCID: PMC6989397
- DOI: 10.1002/jia2.25441
A longitudinal analysis of the completeness of maternal HIV testing, including repeat testing in Cape Town, South Africa
Abstract
Introduction: The virtual elimination of mother-to-child transmission of HIV cannot be achieved without complete maternal HIV testing. The World Health Organization recommends that women in high HIV prevalent settings repeat HIV testing in the third trimester, and at delivery or directly thereafter. The Western Cape Province (South Africa) prevention of mother-to-child transmission (PMTCT) guidelines recommend a repeat maternal HIV test between 32 and 34 weeks gestation and at delivery in addition to testing at the first antenatal visit (ideally <20 weeks gestation). There are few published longitudinal studies on the uptake of initial and repeated maternal HIV testing programmes in sub-Saharan Africa. We aimed to investigate the implementation of initial and repeat maternal HIV testing guidelines in Cape Town, South Africa.
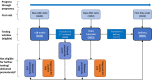
Methods: Between 2013 and 2016 we established an electronic PMTCT register that consolidated routine data from a primary healthcare facility and its secondary and tertiary referral sites in Cape Town. This provided a longitudinal record for each participant, from first antenatal visit to delivery. Utilizing these data, we conducted a retrospective analysis investigating the completeness of maternal HIV testing according to the PMTCT HIV testing guidelines in Cape Town, and predictors of complete testing, from 2014 to 2016.
Results: Among 8558 enrolled pregnant women, 7213 (84%) were not known to be HIV positive at their first visit and thus eligible for HIV testing; 91% of them received ≥1 HIV test during pregnancy/delivery. Testing at the first visit was 98% among the 85% of women who attended antenatal care. Among women eligible to receive all three recommended HIV tests, only 11% achieved all three tests. Delivery HIV testing completion among all women without an HIV-positive diagnosis was 23%. HIV prevalence at delivery was 21% and HIV incidence between first visit and delivery in those with ≥2 HIV tests was 0.2%. Women who enrolled after 2014 were more likely to receive the three recommended tests (aOR: 1.41; 95% CI: 1.10 to 1.81) and retest at delivery (aOR: 1.20; 95% CI: 1.05 to 1.39).
Conclusions: Implementation of maternal HIV testing in Cape Town improved between 2014 and 2016 but major gaps remain, particularly at delivery.
Keywords: HIV; PMTCT; South Africa; guideline implementation; maternal HIV testing; repeat testing.
© 2020 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Figures
References
-
- UNAIDS . 2014 PROGRESS REPORT ON THE GLOBAL PLAN towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. [Internet]. 2014. [cited 2018 Jul 3]. Available from: http://www.unaids.org/.../unaids/.../2013/gr2013/UNAIDS_Global_Report_20...
-
- UNAIDS, PEPFAR . UNAIDS and PEPFAR announce dramatic reductions in new HIV infections among children in the 21 countries most affected by HIV in Africa [Internet]. 2016. [cited 2018 Jul 2]. p. 1–3. Available from: http://www.unaids.org/sites/default/files/20160608_PR_GlobalPlan_en.pdf
-
- Stinson K, Boulle A, Smith PJ, Stringer EM, Stringer JS, Coetzee D. Coverage of the prevention of mother‐to‐child transmission program in the Western Cape, South Africa using cord blood surveillance. J Acquir Immune Defic Syndr. 2012. [cited 2018 Apr 4];60(2):199–204. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22343175 - PubMed
-
- Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems' performance on mother‐to‐child transmission of HIV. J Acquir Immune Defic Syndr. 2011. [cited 2018 Apr 2];56(2):45–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21084998 - PubMed
-
- Centers for Disease Control and Prevention (CDC) . Impact of an innovative approach to prevent mother‐to‐child transmission of HIV – Malawi, July 2011‐September 2012. MMWR Morb Mortal Wkly Rep. 2013. [cited 2018 Sep 2];62(8):148–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23446512%255Cnhttp://www.ncbi.nlm.nih... - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

