Staphylococcus aureus facilitates its survival in bovine macrophages by blocking autophagic flux
- PMID: 31997584
- PMCID: PMC7131951
- DOI: 10.1111/jcmm.15027
Staphylococcus aureus facilitates its survival in bovine macrophages by blocking autophagic flux
Abstract
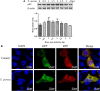
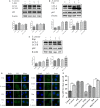
Staphylococcus aureus is a pathogen that is the causative agent of several human and veterinary infections and plays a critical role in the clinical and subclinical mastitis of cattle. Autophagy is a conserved pathogen defence mechanism in eukaryotes. Studies have reported that S aureus can subvert autophagy and survive in cells. Staphylococcus aureus survival in cells is an important cause of chronic persistent mastitis infection. However, it is unclear whether S aureus can escape autophagy in innate immune cells. In this study, initiation of autophagy due to the presence of S aureus was detected in bovine macrophages. We observed autophagic vacuoles increased after S aureus infection of bovine macrophages by transmission electron microscopy (TEM). It was also found that S aureus-infected bovine macrophages increased the expression of LC3 at different times(0, 0.5, 1, 1.5, 2, 2.5, 3 and 4 hours). Data also showed the accumulation of p62 induced by S aureus infection. Application of autophagy regulatory agents showed that the degradation of p62 was blocked in S aureus induced bovine macrophages. In addition, we also found that the accumulation of autophagosomes promotes S aureus to survive in macrophage cells. In conclusion, this study indicates that autophagy occurs in S aureus-infected bovine macrophages but is blocked at a later stage of autophagy. The accumulation of autophagosomes facilitates the survival of S aureus in bovine macrophages. These findings provide new insights into the interaction of S aureus with autophagy in bovine macrophages.
Keywords: Staphylococcus aureus; autophagy; bovine macrophage.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The author(s) declare no potential conflicts of interests with respect to the research, authorship and/or publication of this article.
Figures



Similar articles
-
Staphylococcus aureus induces autophagy in bovine mammary epithelial cells and the formation of autophagosomes facilitates intracellular replication of Staph. aureus.J Dairy Sci. 2019 Sep;102(9):8264-8272. doi: 10.3168/jds.2019-16414. Epub 2019 Jun 27. J Dairy Sci. 2019. PMID: 31255277
-
Staphylococcus aureus Avoids Autophagy Clearance of Bovine Mammary Epithelial Cells by Impairing Lysosomal Function.Front Immunol. 2020 May 5;11:746. doi: 10.3389/fimmu.2020.00746. eCollection 2020. Front Immunol. 2020. PMID: 32431700 Free PMC article.
-
Intracellular Staphylococcus aureus inhibits autophagy of bovine mammary epithelial cells through activating p38α.J Dairy Res. 2021 Aug;88(3):293-301. doi: 10.1017/S0022029921000649. J Dairy Res. 2021. PMID: 34425921
-
Staphylococcus aureus persistence properties associated with bovine mastitis and alternative therapeutic modalities.J Appl Microbiol. 2020 Nov;129(5):1102-1119. doi: 10.1111/jam.14706. Epub 2020 Jun 8. J Appl Microbiol. 2020. PMID: 32416020 Review.
-
Coagulase-negative staphylococci as cause of bovine mastitis- not so different from Staphylococcus aureus?Vet Microbiol. 2009 Feb 16;134(1-2):29-36. doi: 10.1016/j.vetmic.2008.09.011. Epub 2008 Sep 11. Vet Microbiol. 2009. PMID: 18977615 Review.
Cited by
-
Rapamycin Exacerbates Staphylococcus aureus Pneumonia by Inhibiting mTOR-RPS6 in Macrophages.J Inflamm Res. 2023 Nov 30;16:5715-5728. doi: 10.2147/JIR.S434483. eCollection 2023. J Inflamm Res. 2023. PMID: 38053607 Free PMC article.
-
Staphylococcal mastitis in dairy cows.Front Vet Sci. 2024 May 28;11:1356259. doi: 10.3389/fvets.2024.1356259. eCollection 2024. Front Vet Sci. 2024. PMID: 38863450 Free PMC article. Review.
-
The Role of Macrophages in Staphylococcus aureus Infection.Front Immunol. 2021 Jan 19;11:620339. doi: 10.3389/fimmu.2020.620339. eCollection 2020. Front Immunol. 2021. PMID: 33542723 Free PMC article. Review.
-
From Inflammation to Fibrosis: Novel Insights into the Roles of High Mobility Group Protein Box 1 in Schistosome-Induced Liver Damage.Pathogens. 2022 Feb 24;11(3):289. doi: 10.3390/pathogens11030289. Pathogens. 2022. PMID: 35335612 Free PMC article. Review.
-
PINK1/Parkin-mediated mitophagy enhances the survival of Staphylococcus aureus in bovine macrophages.J Cell Mol Med. 2023 Feb;27(3):412-421. doi: 10.1111/jcmm.17664. Epub 2023 Jan 9. J Cell Mol Med. 2023. PMID: 36625039 Free PMC article.
References
-
- Viguier C, Arora S, Gilmartin N, et al. Mastitis detection: current trends and future perspectives. Trends Biotechnol. 2009;27:486‐493. - PubMed
-
- Hogeveen H, Huijps K, Lam TJ. Economic aspects of mastitis: new developments. N Z Vet J. 2011;59:16‐23. - PubMed
-
- Vliegher SD, Fox LK, Piepers S, et al. Invited review: mastitis in dairy heifers: nature of the disease, potential impact, prevention, and control. J Dairy Sci. 2012;95:1025‐1040. - PubMed
-
- Dufour S, Dohoo IR, Barkema HW, et al. Manageable risk factors associated with the lactational incidence, elimination, and prevalence of Staphylococcus aureus intramammary infections in dairy cows. J Dairy Sci. 2012;95:1283‐1300. - PubMed
-
- Finlay BB, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276(5313):718‐725. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

