Role of the metabolism of branched-chain amino acids in the development of Alzheimer's disease and other metabolic disorders
- PMID: 31997805
- PMCID: PMC7059578
- DOI: 10.4103/1673-5374.274328
Role of the metabolism of branched-chain amino acids in the development of Alzheimer's disease and other metabolic disorders
Abstract
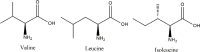
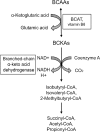
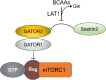
Alzheimer's disease is an incurable chronic neurodegenerative disorder and the leading cause of dementia, imposing a growing economic burden upon society. The disease progression is associated with gradual deposition of amyloid plaques and the formation of neurofibrillary tangles within the brain parenchyma, yet severe dementia is the culminating phase of the enduring pathology. Converging evidence suggests that Alzheimer's disease-related cognitive decline is the outcome of an extremely complex and persistent pathophysiological process. The disease is characterized by distinctive abnormalities apparent at systemic, histological, macromolecular, and biochemical levels. Moreover, besides the well-defined and self-evident characteristic profuse neurofibrillary tangles, dystrophic neurites, and amyloid-beta deposits, the Alzheimer's disease-associated pathology includes neuroinflammation, substantial neuronal loss, apoptosis, extensive DNA damage, considerable mitochondrial malfunction, compromised energy metabolism, and chronic oxidative stress. Likewise, distinctive metabolic dysfunction has been named a leading cause and a hallmark of Alzheimer's disease that is apparent decades prior to disease manifestation. State-of-the-art metabolomics studies demonstrate that altered branched-chain amino acids (BCAAs) metabolism accompanies Alzheimer's disease development. Lower plasma valine levels are correlated with accelerated cognitive decline, and, conversely, an increase in valine concentration is associated with reduced risk of Alzheimer's disease. Additionally, a clear BCAAs-related metabolic signature has been identified in subjects with obesity, diabetes, and atherosclerosis. Also, arginine metabolism is dramatically altered in Alzheimer's disease human brains and animal models. Accordingly, a potential role of the urea cycle in the Alzheimer's disease development has been hypothesized, and preclinical studies utilizing intervention in the urea cycle and/or BCAAs metabolism have demonstrated clinical potential. Continual failures to offer a competent treatment strategy directed against amyloid-beta or Tau proteins-related lesions, which could face all challenges of the multifaceted Alzheimer's disease pathology, led to the hypothesis that hyperphosphorylated Tau and deposited amyloid-beta proteins are just hallmarks or epiphenomena, but not the ultimate causes of Alzheimer's disease. Therefore, approaches targeting amyloid-beta or Tau are not adequate to cure the disease. Accordingly, the modern scientific vision of Alzheimer's disease etiology and pathogenesis must reach beyond the hallmarks, and look for alternative strategies and areas of research.
Keywords: BCAAs; arginase; arginine; branched-chain aminotransferase; dementia; mTOR; norvaline; urea cycle; valine.
Conflict of interest statement
None
Figures



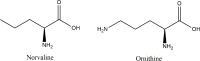
References
-
- Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: A population-based neuropathologic study. Neurology. 2010;75:1195–1202. - PubMed
-
- Albrecht J, Zielińska M. Exchange-mode glutamine transport across CNS cell membranes. Neuropharmacology. 2019 doi: 101016/jneuropharm201903003. - PubMed
-
- Alford S, Patel D, Perakakis N, Mantzoros CS. Obesity as a risk factor for Alzheimer’s disease: weighing the evidence. Obes Rev. 2018;19:269–280. - PubMed
-
- Alzheimer’s Association 2016. Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

