Gastric floating sustained-release tablet for dihydromyricetin: Development, characterization, and pharmacokinetics study
- PMID: 31997907
- PMCID: PMC6978620
- DOI: 10.1016/j.jsps.2019.08.002
Gastric floating sustained-release tablet for dihydromyricetin: Development, characterization, and pharmacokinetics study
Abstract
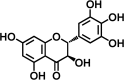
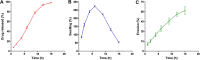
Dihydromyricetin (DHM) is a natural dihydroflavonol compound with quite a number of important pharmacological properties. However, its low solubility in water and poor stability in aqueous environment, have compromised drug efficacy of DHM, thus hindering its clinical use. The present study was to develop DHM-loaded gastric floating sustained-release tablet (DHM-GFT) to improve the bioavailability of DHM. DHM-GFT was prepared via powder direct compression. The formulation of tablet was optimized in terms of the floating ability and drug release rate. The optimized DHM-GFT exhibited short floating lag time of less than 10 s and long floating duration of over 12 h in acidic medium. It had a 12-hour sustained release of DHM, which proved its potential to develop as a twice-a-day dosing preparation. The physicochemical properties of DHM-GFT well satisfied the pharmacopoeial requirements. In addition, the results from pharmacokinetic studies demonstrated that, DHM-GFT could considerably prolong the in vivo residence time of drug and improve the bioavailability via good gastric floating ability and sustained drug release when compared to DHM powder. Therefore, DHM-GFT is promising to promote the application of DHM and merits studies for further development.
Keywords: Bioavailability; Dihydromyricetin; Gastric floating tablet; Pharmacokinetics; Stability; Sustained release.
© 2019 The Authors.
Figures




References
-
- Acharya S., Patra S., Pani N.R. Optimization of HPMC and carbopol concentrations in non-effervescent floating tablet through factorial design. Carbohyd. Polym. 2014;102:360–368. - PubMed
-
- Asadian-Ardakani V., Saber-Samandari S., Saber-Samandari S. The effect of hydroxyapatite in biopolymer-based scaffolds on release of naproxen sodium. J. Biomed. Mater. Res. A. 2016;104(12):2992–3003. - PubMed
-
- Chinese Pharmacopoeia Commission . 2015 ed. China Medical Science Press; Beijing: 2015. Pharmacopoeia of the People's Republic of China.
-
- Fu J., Yin H., Yu X., Xie C., Jiang H., Jin Y., Sheng F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. Int. J. Pharmaceut. 2018;549(1–2):370–379. - PubMed
LinkOut - more resources
Full Text Sources

