The Application of EEG Mu Rhythm Measures to Neurophysiological Research in Stuttering
- PMID: 31998103
- PMCID: PMC6965028
- DOI: 10.3389/fnhum.2019.00458
The Application of EEG Mu Rhythm Measures to Neurophysiological Research in Stuttering
Abstract
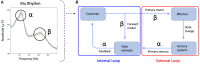
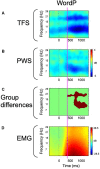
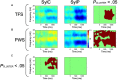
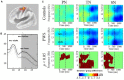
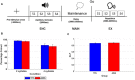
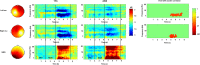
Deficits in basal ganglia-based inhibitory and timing circuits along with sensorimotor internal modeling mechanisms are thought to underlie stuttering. However, much remains to be learned regarding the precise manner how these deficits contribute to disrupting both speech and cognitive functions in those who stutter. Herein, we examine the suitability of electroencephalographic (EEG) mu rhythms for addressing these deficits. We review some previous findings of mu rhythm activity differentiating stuttering from non-stuttering individuals and present some new preliminary findings capturing stuttering-related deficits in working memory. Mu rhythms are characterized by spectral peaks in alpha (8-13 Hz) and beta (14-25 Hz) frequency bands (mu-alpha and mu-beta). They emanate from premotor/motor regions and are influenced by basal ganglia and sensorimotor function. More specifically, alpha peaks (mu-alpha) are sensitive to basal ganglia-based inhibitory signals and sensory-to-motor feedback. Beta peaks (mu-beta) are sensitive to changes in timing and capture motor-to-sensory (i.e., forward model) projections. Observing simultaneous changes in mu-alpha and mu-beta across the time-course of specific events provides a rich window for observing neurophysiological deficits associated with stuttering in both speech and cognitive tasks and can provide a better understanding of the functional relationship between these stuttering symptoms. We review how independent component analysis (ICA) can extract mu rhythms from raw EEG signals in speech production tasks, such that changes in alpha and beta power are mapped to myogenic activity from articulators. We review findings from speech production and auditory discrimination tasks demonstrating that mu-alpha and mu-beta are highly sensitive to capturing sensorimotor and basal ganglia deficits associated with stuttering with high temporal precision. Novel findings from a non-word repetition (working memory) task are also included. They show reduced mu-alpha suppression in a stuttering group compared to a typically fluent group. Finally, we review current limitations and directions for future research.
Keywords: basal ganglia; internal models; mu rhythm; sensorimotor integration; speech perception; speech production; stuttering; working memory.
Copyright © 2020 Jenson, Bowers, Hudock and Saltuklaroglu.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

