Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain
- PMID: 31998120
- PMCID: PMC6966240
- DOI: 10.3389/fnagi.2019.00373
Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain
Abstract
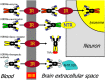
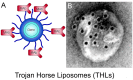
Alzheimer's disease (AD) and treatment of the brain in aging require the development of new biologic drugs, such as recombinant proteins or gene therapies. Biologics are large molecule therapeutics that do not cross the blood-brain barrier (BBB). BBB drug delivery is the limiting factor in the future development of new therapeutics for the brain. The delivery of recombinant protein or gene medicines to the brain is a binary process: either the brain drug developer re-engineers the biologic with BBB drug delivery technology, or goes forward with brain drug development in the absence of a BBB delivery platform. The presence of BBB delivery technology allows for engineering the therapeutic to enable entry into the brain across the BBB from blood. Brain drug development may still take place in the absence of BBB delivery technology, but with a reliance on approaches that have rarely led to FDA approval, e.g., CSF injection, stem cells, small molecules, and others. CSF injection of drug is the most widely practiced approach to brain delivery that bypasses the BBB. However, drug injection into the CSF results in limited drug penetration to the brain parenchyma, owing to the rapid export of CSF from the brain to blood. A CSF injection of a drug is equivalent to a slow intravenous (IV) infusion of the pharmaceutical. Given the profound effect the existence of the BBB has on brain drug development, future drug or gene development for the brain will be accelerated by future advances in BBB delivery technology in parallel with new drug discovery.
Keywords: Alzheimer’s disease; Trojan horse; blood-brain barrier; cerebrospinal fluid; endothelium.
Copyright © 2020 Pardridge.
Figures




References
-
- Abe T., Terada K., Wakimoto H., Inoue R., Tyminski E., Bookstein R., et al. . (2003). PTEN decreases in vivo vascularization of experimental gliomas in spite of proangiogenic stimuli. Cancer Res. 63, 2300–2305. - PubMed
-
- Agarwal S., Uchida Y., Mittapalli R. K., Sane R., Terasaki T., Elmquist W. F. (2012). Quantitative proteomics of transporter expression in brain capillary endothelial cells isolated from P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp) and P-gp/Bcrp knockout mice. Drug Metab. Dispos. 40, 1164–1169. 10.1124/dmd.112.044719 - DOI - PMC - PubMed
-
- Almaguer-Melian W., Merceron-Martínez D., Pavón-Fuentes N., Alberti-Amador E., Leon-Martinez R., Ledon N., et al. . (2015). Erythropoietin promotes neural plasticity and spatial memory recovery in fimbria-fornix-lesioned rats. Neurorehabil. Neural Repair 29, 979–988. 10.1177/1545968315572389 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

