Acidic Microenvironment Regulates the Severity of Hepatic Ischemia/Reperfusion Injury by Modulating the Generation and Function of Tregs via the PI3K-mTOR Pathway
- PMID: 31998287
- PMCID: PMC6962105
- DOI: 10.3389/fimmu.2019.02945
Acidic Microenvironment Regulates the Severity of Hepatic Ischemia/Reperfusion Injury by Modulating the Generation and Function of Tregs via the PI3K-mTOR Pathway
Abstract
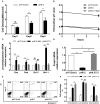
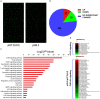
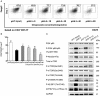
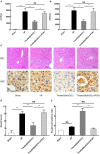
Hepatic ischemia/reperfusion injury (HIRI) is a major cause of liver dysfunction and even liver failure after liver transplantation and hepatectomy. One of the critical mechanisms that lead to HIRI is an acidic microenvironment, which develops due to the accumulation of high acid-like substances such as lactic acid and ketone bodies. Previous studies have shown that the adoptive transfer of induced regulatory T cells (iTregs) attenuates HIRI; however, little is known about the function of Tregs in the acidic microenvironment of a HIRI model. In the present study, we examined the effect of acidic microenvironment on Tregs in vitro and in vivo. Here, we report that microenvironment acidification and dysfunction of the liver is induced during HIRI in humans and mice and that an acidic microenvironment can inhibit the generation and function of CD4+CD25+Foxp3+ iTregs via the PI3K/Akt/mTOR signaling pathway. By contrast, the reversal of the acidic microenvironment restored Foxp3 expression and iTreg function. In addition, the results of cell culture in vitro indicated that the proton pump inhibitor omeprazole improves decreased iTreg differentiation caused by the acidic microenvironment, suggesting the potential clinical use of proton pump inhibitors as immunoregulatory therapy in the treatment of HIRI. Furthermore, our findings demonstrate that buffering the acidic microenvironment to attenuate HIRI in mice has an inseparable relationship with Tregs. Thus, an acidic microenvironment is a key regulator in HIRI, involved in modulating the generation and function of Tregs.
Keywords: Treg; acidic microenvironment; hepatic ischemia/reperfusion injury; immune cells; signaling.
Copyright © 2020 Gan, Zhang, Gu, Ju, Wu, Wang, Peng, Qiu, Zhou, Cheng and Lu.
Figures




References
-
- Li J, Li RJ, Lv GY, Liu HQ. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. (2015) 19:2036–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

