Filtered Kombucha tea ameliorates the leaky gut syndrome in young and old mice model of colitis
- PMID: 31998457
- PMCID: PMC6885390
- DOI: 10.22038/ijbms.2019.36189.8622
Filtered Kombucha tea ameliorates the leaky gut syndrome in young and old mice model of colitis
Abstract
Objectives: Zonula occludens proteins (ZO-1 and ZO-2) are important intracellular tight junction (TJ)-associated proteins that link the cell cytoskeleton to the trans-membrane TJ proteins. Destruction of TJ proteins is called the "leaky gut syndrome" and has been observed in some of the gastrointestinal diseases such as the inflammatory bowel disease (IBD). So, therapeutic approaches aim to restore the expression of TJ proteins and reduce intestinal permeability. Healing effect of Kombucha tea (KT), so-called long-life mushroom, on the gastrointestinal system, particularly its extraordinary healing effects on intestinal ulcers has been purported traditionally and rarely reported scientifically. This study aimed to investigate the therapeutic effect of filtered KT (fKT) in young and old mice model of colitis.
Materials and methods: Leaky gut was induced in two groups of young and old age using dextran sodium sulfate in drinking water for seven days. Then, fKT was administered to the mice affected by colitis and compared with the age-matched normal and untreated animals with colitis.
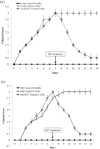
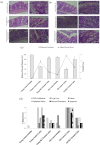
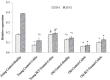
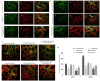
Results: Survival rate of the fKT-treated young and old animals with colitis increased and weight loss decreased. Accordingly, digestive disorders characterized by bleeding and diarrhea were improved in fKT-treated mice. Molecular and histological examination indicated that expression of ZO-1 and ZO-2 was significantly improved in fKT-treated mice.
Conclusion: Our results suggest KT as a promising therapeutic candidate to reduce intestinal permeability. Young animals with colitis showed more severe clinical signs and less survival rate than old mice with colitis, but this group responded better to fKT treatment than the old mice.
Keywords: Age; Colitis; Kombucha tea; Leaky gut; ZO-1; ZO-2.
Conflict of interest statement
The authors have no competing interests.
Figures




References
-
- González-Mariscal L, Betanzos A, Nava P, Jaramillo BE. [Tight junction proteins]; ProgBiophysMolBiol. 2003 81:1–44. - PubMed
LinkOut - more resources
Full Text Sources
