Distinct Immune Responses Elicited From Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated With Optimal and Non-optimal Vaginal Microbiota
- PMID: 31998660
- PMCID: PMC6965070
- DOI: 10.3389/fcimb.2019.00446
Distinct Immune Responses Elicited From Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated With Optimal and Non-optimal Vaginal Microbiota
Abstract
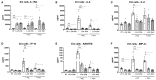
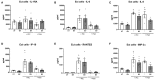
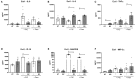
Non-optimal vaginal microbiota, as observed in bacterial vaginosis (BV), is typically characterized by a depletion of beneficial lactobacilli and an abundance of numerous anaerobes. These non-optimal conditions are associated with subclinical cervicovaginal inflammation and an increased risk of HIV infection compared to women colonized with optimal vaginal microbiota dominated by lactobacilli. Lactic acid (LA) is a major organic acid metabolite produced by vaginal lactobacilli that elicits anti-inflammatory effects from cervicovaginal epithelial cells and is dramatically depleted during BV. However, it is unclear if LA retains its anti-inflammatory activity in the presence of vaginal microbiota metabolites comprising short chain fatty acids (SCFAs) and succinic acid, which are also produced by an optimal vaginal microbiota. Furthermore, the immunomodulatory effect of SCFAs and succinic acid on cervicovaginal epithelial cells at higher concentrations present during BV is unknown. Here we report that in the presence of physiologically relevant concentrations of SCFAs and succinic acid at pH 3.9 (as found in women with lactobacillus-dominated microbiota) LA induced an anti-inflammatory state in cervicovaginal epithelial cells and inhibited inflammation elicited by the toll-like receptor (TLR) agonists polyinosinic:polycytidylic acid and Pam3CSK4. When cervicovaginal epithelial cells were treated with a vaginal microbiota metabolite mixture representative of BV, containing a lower concentration of LA but higher concentrations of SCFA/succinic acid at pH 7, no anti-inflammatory was observed. Rather, the vaginal microbiota metabolite mixture representative of BV dysregulated the immune response of cervicovaginal epithelial cells during prolonged and sustained treatments. This was evidenced by increased basal and TLR-induced production of pro-inflammatory cytokines including tumor necrosis factor-α, but decreased basal production of chemokines including RANTES and IP-10. Further characterization of individual components of the BV vaginal microbiota mixture suggested that acetic acid is an important vaginal microbiota metabolite capable of eliciting diverse immunomodulatory effects on a range of cervicovaginal epithelial cell targets. These findings indicate that elevated levels of SCFAs are a potential source of cervicovaginal inflammation in women experiencing BV, and support the unique anti-inflammatory properties of LA on cervicovaginal epithelial cells as well as a role for LA or LA-producing lactobacilli to reverse genital inflammation associated with increased HIV risk.
Keywords: bacterial vaginosis; cervicovaginal epithelium; inflammation; lactic acid; short chain fatty acids; succinic acid; vaginal microbiota metabolites.
Copyright © 2020 Delgado-Diaz, Tyssen, Hayward, Gugasyan, Hearps and Tachedjian.
Figures
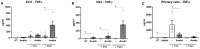
References
-
- Aldunate M., Srbinovski D., Hearps A. C., Latham C. F., Ramsland P. A., Gugasyan R., et al. (2015). Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 6:164. 10.3389/fphys.2015.00164 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

