Convergence of Scaffold-Guided Bone Reconstruction and Surgical Vascularization Strategies-A Quest for Regenerative Matching Axial Vascularization
- PMID: 31998712
- PMCID: PMC6967032
- DOI: 10.3389/fbioe.2019.00448
Convergence of Scaffold-Guided Bone Reconstruction and Surgical Vascularization Strategies-A Quest for Regenerative Matching Axial Vascularization
Abstract
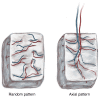
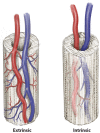
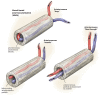
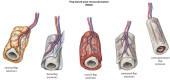
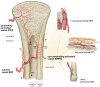
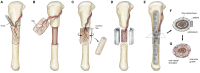
The prevalent challenge facing tissue engineering today is the lack of adequate vascularization to support the growth, function, and viability of tissue engineered constructs (TECs) that require blood vessel supply. The research and clinical community rely on the increasing knowledge of angiogenic and vasculogenic processes to stimulate a clinically-relevant vascular network formation within TECs. The regenerative matching axial vascularization approach presented in this manuscript incorporates the advantages of flap-based techniques for neo-vascularization yet also harnesses the in vivo bioreactor principle in a more directed "like for like" approach to further assist regeneration of the specific tissue type that is lost, such as a corticoperiosteal flap in critical sized bone defect reconstruction.
Keywords: blood vessel analysis; bone; regeneration; tissue engineering; vascularization.
Copyright © 2020 Sparks, Savi, Saifzadeh, Schuetz, Wagels and Hutmacher.
Figures



References
-
- Arkudas A., Beier J. P., Heidner K., Tjiawi J., Polykandriotis E., Srour S., et al. (2007). Axial prevascularization of porous matrices using an arteriovenous loop promotes survival and differentiation of transplanted autologous osteoblasts. Tissue Eng. 13, 1549–1560. 10.1089/ten.2006.0387 - DOI - PubMed
-
- Arkudas A., Beier J. P., Pryymachuk G., Hoereth T., Bleiziffer O., Polykandriotis E., et al. (2010). Automatic quantitative micro-computed tomography evaluation of angiogenesis in an axially vascularized tissue-engineered bone construct. Tissue Eng. Part C Methods 16, 1503–1514. 10.1089/ten.tec.2010.0016 - DOI - PubMed
LinkOut - more resources
Full Text Sources

