TNFα Induces Müller Glia to Transition From Non-proliferative Gliosis to a Regenerative Response in Mutant Zebrafish Presenting Chronic Photoreceptor Degeneration
- PMID: 31998714
- PMCID: PMC6962764
- DOI: 10.3389/fcell.2019.00296
TNFα Induces Müller Glia to Transition From Non-proliferative Gliosis to a Regenerative Response in Mutant Zebrafish Presenting Chronic Photoreceptor Degeneration
Abstract
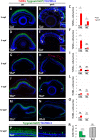
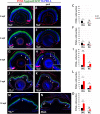
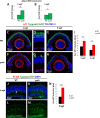
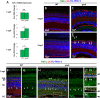
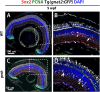
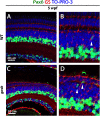
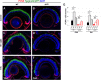
Unlike mammals, zebrafish have the capacity to regenerate neurons in response to damage. Most zebrafish retinal injury models employ acute damage, which is unlike the chronic, gradual damage that occurs in human retinal diseases. Here, we studied the regenerative response in the zebrafish aipl1b mutant, gold rush (gosh). In gosh mutants, both cones and rods degenerate by 3 weeks post-fertilization (wpf). Müller glia do not exhibit a regenerative response by 3 wpf; however, they do present non-proliferative gliosis. Only at 5 wpf, is proliferation of Müller cells and rod precursor cells activated. Rods start to recover at 5 wpf and by 12 wpf they reach a level of recovery comparable to wild type, but cones remain absent in the adult stage. TNFα was detected in degenerating cones at 5-7 wpf and in Müller glia at 7 wpf in gosh mutants. At 5 wpf, proliferating Müller glia express Sox2, followed by Pax6 expression in neuronal progenitor cells (NPCs), confirming that the neuronal regeneration program is activated in gosh mutants after 5 wpf. Although acute light-induced damage did not activate proliferation of Müller glia, TNFα injection caused Müller glia to commence a proliferative response at 3 wpf in gosh mutants. These results suggest that Müller glia transition from non-proliferative gliosis to a regenerative state in gosh mutants, and that ectopic introduction of TNFα promotes this Müller cell transition even at 3 wpf. Thus, zebrafish gosh mutants provide a useful model to investigate mechanisms underlying retinal regeneration in a chronic photoreceptor degeneration model.
Keywords: Aipl1; Müller glia; genetic mutant; photoreceptor degeneration; regeneration; rod precursors; zebrafish.
Copyright © 2019 Iribarne, Hyde and Masai.
Figures
References
-
- Conner C., Ackerman K. M., Lahne M., Hobgood J. S., Hyde D. R. (2014). Repressing notch signaling and expressing TNFα are sufficient to mimic retinal regeneration by inducing Muller glial proliferation to generate committed progenitor cells. J. Neurosci. 34 14403–14419. 10.1523/JNEUROSCI.0498-14.2014 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

