Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach?
- PMID: 31998852
- PMCID: PMC6978558
- DOI: 10.1016/j.jacbts.2019.10.008
Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach?
Abstract
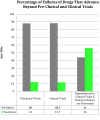
Animal testing is used in pharmaceutical and industrial research to predict human toxicity, and yet analysis suggests that animal models are poor predictors of drug safety in humans. The cost of animal research is high-in dollars, delays in drug approval, and in the loss of potentially beneficial drugs for human use. Human subjects have been harmed in the clinical testing of drugs that were deemed safe by animal studies. Increasingly, investigators are questioning the scientific merit of animal research. This review discusses issues in using animals to predict human toxicity in pharmaceutical development. Part 1 focuses on scientific concerns over the validity of animal research. Part 2 will discuss alternatives to animal research and their validation and use in production of human pharmaceuticals.
Keywords: FDA, U.S. Food and Drug Administration; LR, likelihood ratio; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; animal research; drug development; toxicity; translational research.
© 2019 The Author.
Figures



References
-
- Wax P.M. Elixirs, diluents and the passage of the 1938 Federal Food, Drug and Cosmetics Act. Ann Intern Med. 1995;122:456–461. - PubMed
-
- National Institutes of Health . U.S. Government Printing Office; Washington, DC: 1949. Regulations and Ethical Guidelines. Reprint from: Trials of War Criminals before the Nuremberg Military Tribunals under Control Council Law no. 10, Vol. 2; pp. 181–182.https://history.nih.gov/research/downloads/nuremberg.pdf Available at:
-
- World Medical Assembly Declaration of Helsinki 1964: Recommendations Guiding Doctors in Clinical Research. World Medical Association. June 1964. https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/do... Available at:
-
- U.S. Food and Drug Administration Investigational New Drug (IND) Application. U.S. Food and Drug Administration. October 5, 2017. https://www.fda.gov/drugs/types-applications/investigational-new-drug-in... Available at:
LinkOut - more resources
Full Text Sources
Other Literature Sources

