Iron(II)-Catalyzed Biomimetic Aerobic Oxidation of Alcohols
- PMID: 31999013
- PMCID: PMC7154773
- DOI: 10.1002/anie.202000054
Iron(II)-Catalyzed Biomimetic Aerobic Oxidation of Alcohols
Abstract
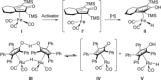
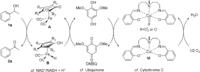
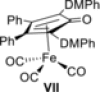
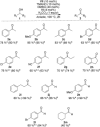
We report the first FeII -catalyzed biomimetic aerobic oxidation of alcohols. The principle of this oxidation, which involves several electron-transfer steps, is reminiscent of biological oxidation in the respiratory chain. The electron transfer from the alcohol to molecular oxygen occurs with the aid of three coupled catalytic redox systems, leading to a low-energy pathway. An iron transfer-hydrogenation complex was utilized as a substrate-selective dehydrogenation catalyst, along with an electron-rich quinone and an oxygen-activating Co(salen)-type complex as electron-transfer mediators. Various primary and secondary alcohols were oxidized in air to the corresponding aldehydes or ketones with this method in good to excellent yields.
Keywords: aerobic oxidation; biomimetic reactions; electron transfer; homogeneous catalysis; iron.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Efficient Aerobic Oxidation of Organic Molecules by Multistep Electron Transfer.Angew Chem Int Ed Engl. 2021 Jul 12;60(29):15686-15704. doi: 10.1002/anie.202012707. Epub 2021 Mar 24. Angew Chem Int Ed Engl. 2021. PMID: 33368909 Free PMC article. Review.
-
Iron(II)-Catalyzed Aerobic Biomimetic Oxidation of Amines using a Hybrid Hydroquinone/Cobalt Catalyst as Electron Transfer Mediator.Angew Chem Int Ed Engl. 2021 May 17;60(21):11819-11823. doi: 10.1002/anie.202102681. Epub 2021 May 4. Angew Chem Int Ed Engl. 2021. PMID: 33725364 Free PMC article.
-
Efficient ruthenium-catalyzed aerobic oxidation of alcohols using a biomimetic coupled catalytic system.J Org Chem. 2002 Mar 8;67(5):1657-62. doi: 10.1021/jo0163750. J Org Chem. 2002. PMID: 11871899
-
Efficient ruthenium-catalyzed aerobic oxidation of amines by using a biomimetic coupled catalytic system.Chemistry. 2005 Apr 8;11(8):2327-34. doi: 10.1002/chem.200401082. Chemistry. 2005. PMID: 15706621
-
Catalytic oxidation of organic substrates by molecular oxygen and hydrogen peroxide by multistep electron transfer--a biomimetic approach.Angew Chem Int Ed Engl. 2008;47(19):3506-23. doi: 10.1002/anie.200700604. Angew Chem Int Ed Engl. 2008. PMID: 18383499 Review.
Cited by
-
Efficient Aerobic Oxidation of Organic Molecules by Multistep Electron Transfer.Angew Chem Int Ed Engl. 2021 Jul 12;60(29):15686-15704. doi: 10.1002/anie.202012707. Epub 2021 Mar 24. Angew Chem Int Ed Engl. 2021. PMID: 33368909 Free PMC article. Review.
-
Mild Aerobic Oxidation of Secondary Alcohols with Water Tolerant Cu(I) Catalyst.ChemSusChem. 2025 Apr 14;18(8):e202402236. doi: 10.1002/cssc.202402236. Epub 2024 Dec 13. ChemSusChem. 2025. PMID: 39601587 Free PMC article.
-
Iron(II)-Catalyzed Aerobic Biomimetic Oxidation of Amines using a Hybrid Hydroquinone/Cobalt Catalyst as Electron Transfer Mediator.Angew Chem Int Ed Engl. 2021 May 17;60(21):11819-11823. doi: 10.1002/anie.202102681. Epub 2021 May 4. Angew Chem Int Ed Engl. 2021. PMID: 33725364 Free PMC article.
-
Heterogeneous Fe-N-C Cocatalyst for Hydroquinone Oxidation Enables Aerobic Oxidation of Primary Alcohols to Aldehydes.Angew Chem Int Ed Engl. 2025 May 12;64(20):e202424778. doi: 10.1002/anie.202424778. Epub 2025 Mar 12. Angew Chem Int Ed Engl. 2025. PMID: 39979222 Free PMC article.
-
Efficient Palladium-Catalyzed Aerobic Oxidative Carbocyclization to Seven-Membered Heterocycles.Chemistry. 2020 Dec 1;26(67):15513-15518. doi: 10.1002/chem.202004265. Epub 2020 Oct 22. Chemistry. 2020. PMID: 32960479 Free PMC article.
References
-
- Modern Oxidation Methods, 2 nd ed. (Ed.: J.-E. Bäckvall), Wiley-VCH, Weinham, 2010.
-
- None
-
- Piera J., Bäckvall J.-E., Angew. Chem. Int. Ed. 2008, 47, 3506–3523; - PubMed
- Angew. Chem. 2008, 120, 3558–3576;
-
- Murahashi S.-I., Zhang D., Chem. Soc. Rev. 2008, 37, 1490–1501; - PubMed
-
- Chen B., Wang L., Gao S., ACS Catal. 2015, 5, 5851–5876.
Publication types
LinkOut - more resources
Full Text Sources

