Cognitive control and automatic interference in mind and brain: A unified model of saccadic inhibition and countermanding
- PMID: 31999149
- PMCID: PMC7315827
- DOI: 10.1037/rev0000181
Cognitive control and automatic interference in mind and brain: A unified model of saccadic inhibition and countermanding
Abstract
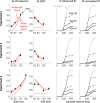
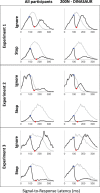
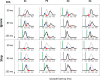
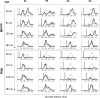
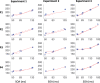
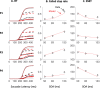
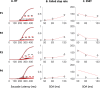
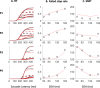
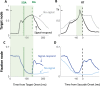
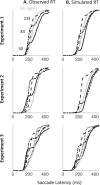
Countermanding behavior has long been seen as a cornerstone of executive control-the human ability to selectively inhibit undesirable responses and change plans. However, scattered evidence implies that stopping behavior is entangled with simpler automatic stimulus-response mechanisms. Here we operationalize this idea by merging the latest conceptualization of saccadic countermanding with a neural network model of visuo-oculomotor behavior that integrates bottom-up and top-down drives. This model accounts for all fundamental qualitative and quantitative features of saccadic countermanding, including neuronal activity. Importantly, it does so by using the same architecture and parameters as basic visually guided behavior and automatic stimulus-driven interference. Using simulations and new data, we compare the temporal dynamics of saccade countermanding with that of saccadic inhibition (SI), a hallmark effect thought to reflect automatic competition within saccade planning areas. We demonstrate how SI accounts for a large proportion of the saccade countermanding process when using visual signals. We conclude that top-down inhibition acts later, piggy-backing on the quicker automatic inhibition. This conceptualization fully accounts for the known effects of signal features and response modalities traditionally used across the countermanding literature. Moreover, it casts different light on the concept of top-down inhibition, its timing and neural underpinning, as well as the interpretation of stop-signal reaction time (RT), the main behavioral measure in the countermanding literature. (PsycInfo Database Record (c) 2020 APA, all rights reserved).
Figures