Differences in inducibility of the latent HIV reservoir in perinatal and adult infection
- PMID: 31999647
- PMCID: PMC7101150
- DOI: 10.1172/jci.insight.134105
Differences in inducibility of the latent HIV reservoir in perinatal and adult infection
Abstract
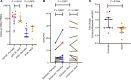
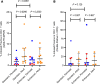
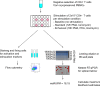
The HIV latent reservoir in resting memory CD4+ T cells precludes cure. Therapeutics to reactivate and eliminate this reservoir are in clinical trials in adults, but not yet in pediatric populations. We determined, ex vivo, the inducibility of the latent reservoir in perinatal infection as compared with adult infections using the Tat/rev induced limiting dilution assay (TILDA), in which a single round (12 hours) of CD4+ T cell stimulation with PMA/ionomycin maximally activates T cells and leads to proviral expression with multiply spliced HIV RNA production. Markers of immune activation and exhaustion were measured to assess interactions with inducibility. Although rates of T cell activation with PMA/ionomycin were similar, the latent reservoir in perinatal infection was slower to reactivate and of lower magnitude compared with adult infection, independent of proviral load. An enhanced TILDA with the addition of phytohemagglutin and a duration of 18 hours augmented proviral expression in perinatal but not adult infection. The baseline HLA-DR+CD4+ T cell level was significantly lower in perinatal compared with adult infections, but not correlated with induced reservoir size. These data support the hypothesis that there are differences in kinetics of latency reversal and baseline immune activation in perinatal compared with adult infections, with implications for latency reversal strategies toward reservoir clearance and remission.
Keywords: AIDS/HIV; Immunology; Molecular biology; T cells.
Conflict of interest statement
Figures



References
-
- [No authors listed]. 2018 Global HIV Statistics — Fact Sheet for World AIDS Day 2019. UNAIDS. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_... Accessed February 20, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

