Landscapes of binding antibody and T-cell responses to pox-protein HIV vaccines in Thais and South Africans
- PMID: 31999736
- PMCID: PMC6992005
- DOI: 10.1371/journal.pone.0226803
Landscapes of binding antibody and T-cell responses to pox-protein HIV vaccines in Thais and South Africans
Abstract
Background: HIV vaccine trials routinely measure multiple vaccine-elicited immune responses to compare regimens and study their potential associations with protection. Here we employ unsupervised learning tools facilitated by a bidirectional power transformation to explore the multivariate binding antibody and T-cell response patterns of immune responses elicited by two pox-protein HIV vaccine regimens. Both regimens utilized a recombinant canarypox vector (ALVAC-HIV) prime and a bivalent recombinant HIV-1 Envelope glycoprotein 120 subunit boost. We hypothesized that within each trial, there were participant subgroups sharing similar immune responses and that their frequencies differed across trials.
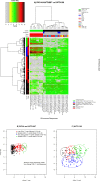
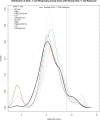
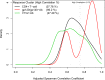
Methods and findings: We analyzed data from three trials-RV144 (NCT00223080), HVTN 097 (NCT02109354), and HVTN 100 (NCT02404311), the latter of which was pivotal in advancing the tested pox-protein HIV vaccine regimen to the HVTN 702 Phase 2b/3 efficacy trial. We found that bivariate CD4+ T-cell and anti-V1V2 IgG/IgG3 antibody response patterns were similar by age, sex-at-birth, and body mass index, but differed for the pox-protein clade AE/B alum-adjuvanted regimen studied in RV144 and HVTN 097 (PAE/B/alum) compared to the pox-protein clade C/C MF59-adjuvanted regimen studied in HVTN 100 (PC/MF59). Specifically, more PAE/B/alum recipients had low CD4+ T-cell and high anti-V1V2 IgG/IgG3 responses, and more PC/MF59 recipients had broad responses of both types. Analyses limited to "vaccine-matched" antigens suggested that some of the differences in responses between the regimens could have been due to antigens in the assays that did not match the vaccine immunogens. Our approach was also useful in identifying subgroups with unusually absent or high co-responses across assay types, flagging individuals for further characterization by functional assays. We also found that co-responses of anti-V1V2 IgG/IgG3 and CD4+ T cells had broad variability. As additional immune response assays are standardized and validated, we anticipate our framework will be increasingly valuable for multivariate analysis.
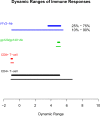
Conclusions: Our approach can be used to advance vaccine development objectives, including the characterization and comparison of candidate vaccine multivariate immune responses and improved design of studies to identify correlates of protection. For instance, results suggested that HVTN 702 will have adequate power to interrogate immune correlates involving anti-V1V2 IgG/IgG3 and CD4+ T-cell co-readouts, but will have lower power to study anti-gp120/gp140 IgG/IgG3 due to their lower dynamic ranges. The findings also generate hypotheses for future testing in experimental and computational analyses aimed at achieving a mechanistic understanding of vaccine-elicited immune response heterogeneity.
Conflict of interest statement
The authors of this manuscript have read the journal's policy and have the following competing interests: LC, ZM, YF, NG, GEG, HEJ, MJM, NR, SCR, NF, GDT, and PBG are recipients of funding from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH), and this publication is a result of activities funded by the NIAID. PBG and GDT are recipients of funding from the Bill and Melinda Gates Foundation, and this publication is a result of activities funded by the BMGF. SR-N is an employee of the Department of Disease Control in the Ministry of Public Heath (Thailand), and this publication is a result of activities conducted through facilities of the Thailand MPH. NLM was an employee of the U.S. Army and MLR is an employee of the Henry Jackson Foundation components of the Military HIV Research Program (MHRP)/Walter Reed Army Institute of Research, which is supported by the US Department of Defense, and this publication is a result of activities partially funded by the DOD. HEJ is a recipient of funding from the National Cancer Institute of the NIH. YH is a recipient of funding from the National Institute of General Medical Sciences of the NIH. AFG is the recipient of funding from the Infectious Disease Research Institute. LC has received grants from Gilead, Sanofi Pasteur Biologics and Immune Design Corp. PBG is the recipient of a contract from Sanofi Pasteur and has served as an unpaid consultant at advisory meetings to Sanofi Pasteur. LNC, ZM, YH, YF, and PBG have received salary support from the contract with Sanofi Pasteur and ZM, YH, YF, and PBG have received support for travel to meetings from Sanofi Pasteur. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures




References
-
- UNAIDS. UNAIDS data 2018. Available from: http://www.unaids.org/en/resources/documents/2018/unaids-data-2018.
-
- Plotkin SA. Increasing Complexity of Vaccine Development. J Infect Dis. 2015;212 Suppl 1:S12–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

