Necroptosis in pancreatic cancer promotes cancer cell migration and invasion by release of CXCL5
- PMID: 31999765
- PMCID: PMC6991976
- DOI: 10.1371/journal.pone.0228015
Necroptosis in pancreatic cancer promotes cancer cell migration and invasion by release of CXCL5
Abstract
Background: Necroptosis is a form of programmed cell death that is accompanied by release of intracellular contents, and reportedly contributes to various diseases. Here, we investigate the significance of necroptosis in pancreatic cancer.
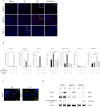
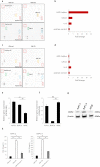
Methods: We used immunohistochemistry and western blot analysis to evaluate expression of the key mediators of necroptosis-receptor-interacting serine/threonine protein kinase 3 (RIP3) and mixed lineage kinase domain-like (MLKL)-in human pancreatic cancer. We also tested the effects of conditioned media (CM) from necroptotic cells on pancreatic cancer cells in Transwell migration and Matrigel invasion assays. Protein array analysis was used to investigate possible mediators derived from necroptotic cells.
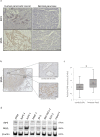
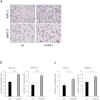
Results: RIP3 and MLKL are highly expressed in human pancreatic cancer tissues compared with normal pancreas. MLKL expression was particularly intense at the tumor invasion front. CM derived from necroptotic cells promoted cancer cell migration and invasion, but not CM derived from apoptotic cells. C-X-C motif chemokine 5 (CXCL5) was upregulated in CM derived from necroptotic cells compared with CM derived from control or apoptotic cells. Moreover, expression of the receptor for CXCL5, C-X-C-motif chemokine receptor-2 (CXCR2), was upregulated in pancreatic cancer cells. Inhibition of CXCR2 suppressed cancer cell migratory and invasive behavior enhanced by necroptosis.
Conclusion: These findings indicate that necroptosis at the pancreatic cancer invasion front can promote cancer cell migration and invasion via the CXCL5-CXCR2 axis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Smac mimetic triggers necroptosis in pancreatic carcinoma cells when caspase activation is blocked.Cancer Lett. 2016 Sep 28;380(1):31-8. doi: 10.1016/j.canlet.2016.05.036. Epub 2016 Jun 3. Cancer Lett. 2016. PMID: 27267809
-
Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer.Am J Pathol. 2011 Mar;178(3):1340-9. doi: 10.1016/j.ajpath.2010.11.058. Am J Pathol. 2011. PMID: 21356384 Free PMC article.
-
Activated CXCL5-CXCR2 axis promotes the migration, invasion and EMT of papillary thyroid carcinoma cells via modulation of β-catenin pathway.Biochimie. 2018 May;148:1-11. doi: 10.1016/j.biochi.2018.02.009. Epub 2018 Feb 20. Biochimie. 2018. PMID: 29471001
-
The role of necroptosis in cancer biology and therapy.Mol Cancer. 2019 May 23;18(1):100. doi: 10.1186/s12943-019-1029-8. Mol Cancer. 2019. PMID: 31122251 Free PMC article. Review.
-
Complex roles of necroptosis in cancer.J Zhejiang Univ Sci B. 2019 May;20(5):399-413. doi: 10.1631/jzus.B1900160. J Zhejiang Univ Sci B. 2019. PMID: 31090266 Free PMC article. Review.
Cited by
-
Single-cell RNA-seq analyses inform necroptosis-associated myeloid lineages influence the immune landscape of pancreas cancer.Front Immunol. 2023 Dec 12;14:1263633. doi: 10.3389/fimmu.2023.1263633. eCollection 2023. Front Immunol. 2023. PMID: 38149248 Free PMC article.
-
A Novel Prognostic Predictor of Immune Microenvironment and Therapeutic Response in Kidney Renal Clear Cell Carcinoma based on Necroptosis-related Gene Signature.Int J Med Sci. 2022 Jan 24;19(2):377-392. doi: 10.7150/ijms.69060. eCollection 2022. Int J Med Sci. 2022. PMID: 35165523 Free PMC article.
-
A Necroptosis-Related lncRNA to Develop a Signature to Predict the Outcome, Immune Landscape, and Chemotherapeutic Responses in Bladder Urothelial Carcinoma.Front Oncol. 2022 Jun 24;12:928204. doi: 10.3389/fonc.2022.928204. eCollection 2022. Front Oncol. 2022. PMID: 35814472 Free PMC article.
-
Predicting the Clinical Outcome of Triple-Negative Breast Cancer Based on the Gene Expression Characteristics of Necroptosis and Different Molecular Subtypes.Stem Cells Int. 2023 Feb 20;2023:8427767. doi: 10.1155/2023/8427767. eCollection 2023. Stem Cells Int. 2023. PMID: 37274025 Free PMC article.
-
The unfolding story of dying tumor cells during cancer treatment.Front Immunol. 2023 Mar 13;14:1073561. doi: 10.3389/fimmu.2023.1073561. eCollection 2023. Front Immunol. 2023. PMID: 36993986 Free PMC article. Review.
References
-
- Garcia-Carbonero N, Li W, Cabeza-Morales M, Martinez-Useros J, Garcia-Foncillas J, Garcia-Carbonero N, et al. New Hope for Pancreatic Ductal Adenocarcinoma Treatment Targeting Endoplasmic Reticulum Stress Response: A Systematic Review. Int J Mol Sci. 2018;19: 2468 10.3390/ijms19092468 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

