HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: A systematic review and meta-analysis
- PMID: 32000054
- PMCID: PMC7230134
- DOI: 10.1016/j.ctrv.2020.101965
HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: A systematic review and meta-analysis
Abstract
Background: HER2-positive (HER2+) breast cancer (BC) comprises all the four PAM50 molecular subtypes. Among these, the HER2-Enriched (HER2-E) appear to be associated with higher pathological complete response (pCR) rates following anti-HER2-based regimens. Here, we present a meta-analysis to validate the association of the HER2-E subtype with pCR following anti-HER2-based neoadjuvant treatments with or without chemotherapy (CT).
Methods: A systematic literature search was performed in February 2019. The primary objective was to compare the association between HER2-E subtype (versus others) and pCR. Selected secondary objectives were to compare the association between 1) HER2-E subtype and pCR in CT-free studies, 2) HER2-E subtype within hormone receptor (HR)-negative and HR+ disease and 3) HR-negative disease (versus HR+) and pCR in all patients and within HER2-E subtype. A random-effect model was applied. The Higgins' I2 was used to quantify heterogeneity.
Results: Sixteen studies were included, 5 of which tested CT-free regimens. HER2-E subtype was significantly associated with pCR in all patients (odds ratio [OR] = 3.50, p < 0.001, I2 = 33%), in HR+ (OR = 3.61, p < 0.001, I2 = 1%) and HR-negative tumors (OR = 2.28, p = 0.01, I2 = 47%). In CT-free studies, HER2-E subtype was associated with pCR in all patients (OR = 5.52, p < 0.001, I2 = 0%) and in HR + disease (OR = 4.08, p = 0.001, I2 = 0%). HR-negative status was significantly associated with pCR compared to HR + status in all patients (OR = 2.41, p < 0.001, I2 = 30%) and within the HER2-E subtype (OR = 1.76, p < 0.001, I2 = 0%).
Conclusions: The HER2-E biomarker identifies patients with a higher likelihood of achieving a pCR following neoadjuvant anti-HER2-based therapy beyond HR status and CT use. Future trial designs to escalate or de-escalate systemic therapy in HER2+ disease should consider this genomic biomarker.
Keywords: Biomarker; Breast cancer; HER2-Enriched; HER2-positive; PAM50; Pathologic complete response.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest FS has declared travel and accommodation expenses paid by Roche, Pfizer and Celgene. SDP has declared honoraria from Roche, Pfizer, Astra-Zeneca, Novartis, Celgene, Eli Lilly, Amgen and Eisai. AP has declared an immediate family member being employed by Novartis, personal honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly and Daiichi Sankyo, travel, accommodations and expenses paid by Daiichi Sankyo, research funding from Roche and Novartis, consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb and patent PCT/EP2016/080056: HER2 AS A PREDICTOR OF RESPONSE TO DUAL HER2 BLOCKADE IN THE ABSENCE OF CYTOTOXIC THERAPY. OTHER AUTHORS CoI. PFC had declared consultant role for Novartis, Eli Lilly, Astra Zeneca and Tesaro, honoraria from BMS, Roche, Eli Lilly, Novartis and AstraZeneca, research funding from Novartis, Roche, BMS, Merck-KGa, Italian Ministry of Health, Veneto Secretary of Health and University of Padova. CMP is an equity stock holder and consultant of BioClassifier LLC and is also listed an inventor on patent applications on the Breast PAM50. LAC has declared that Companies who have provided funds to her institution in the past 1–2 years either for her service on advisory/consultative programs or sponsored research were Genentech, Roche, Novartis, Seattle Genetics, G1 Therapeutics, Immunomedics and Innocrin.SP has received honoraria for talks and travel grants from Roche outside of the submitted work and serves as an advisor/consultant to Polyphor. RS has declared research funding from AstraZeneca, GlaxoSmithKline, Gilead Sciences, and PUMA Biotechnology, and consulting/advisory role with compensation for Macrogenics, and Eli Lilly. CKO has declared research funding from AstraZeneca and GlaxoSmithKline, advisory boards for Tolmar Pharmaceuticals, Genentech, and AstraZeneca, DMC for Eli Lilly and stockholder of GeneTex. MFR has declared research funding from GlaxoSmithKline and Genentech. JCB reports employment and stocks with Novartis. The other authors have nothing to declare.
Figures


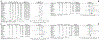


References
-
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(20):2105–2122. doi:10.1200/JCO.2018.77.8738 - DOI - PubMed
-
- Swain SM, Kim S-B, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi:10.1016/S1470-2045(13)70130-X - DOI - PMC - PubMed
-
- Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688–1700. doi:10.1016/S1470-2045(17)30717-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

