Twenty-five-Year Follow-up of a Prospective Randomized Trial Comparing Preoperative Versus Postoperative FLAC/Granulocyte Colony-Stimulating Factor Chemotherapy for Stage II Breast Cancer
- PMID: 32000167
- PMCID: PMC7183875
- DOI: 10.1097/COC.0000000000000667
Twenty-five-Year Follow-up of a Prospective Randomized Trial Comparing Preoperative Versus Postoperative FLAC/Granulocyte Colony-Stimulating Factor Chemotherapy for Stage II Breast Cancer
Abstract
Background: Preoperative chemotherapy is important in the management of women with breast cancer, with the ability to downstage the breast primary tumor and axillary lymph nodes. Long-term studies are needed to identify late toxicities, recurrence patterns, and equivalency with postoperative chemotherapy for recurrence-free survival (RFS) and overall survival (OS).
Patients and methods: We conducted a single-institution prospective randomized control trial comparing preoperative or postoperative fluorouracil, leucovorin calcium, doxorubicin, and cyclophosphamides/granulocyte colony-stimulating factor chemotherapy for women with untreated clinical stage II (T1N1, T2N0, and T2N1) breast cancer. Long-term follow-up was conducted to define toxicities, recurrence patterns and RFS and OS.
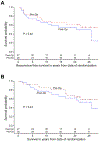
Results: Fifty-three women with clinical stage II breast cancer were randomized, 26 patients to receive preoperative chemotherapy and 27 to receive postoperative chemotherapy. Long-term follow-up, with a median of 25.3 years, was obtained. Local or systemic recurrence occurred in 8 women in the preoperative group and in 10 women in the postoperative group, and recurrences were predominantly within 10 years of treatment. Late toxicities included local upper extremity paresthesia's, upper extremity edema and congestive heart failure in 1 patient each. Analysis revealed no difference in RFS (20-year RFS probabilities; preoperative: 61.3%, postoperative: 54.7%, P=0.42), or in OS between the 2 treatment groups (20-year probabilities, preoperative: 64.6%, postoperative: 62.2%, P=0.44). Twenty-five of 53 patients (47%) were alive and without disease at this follow-up.
Conclusion: Twenty-five-year follow-up for this prospective randomized trial confirms the equivalency of preoperative versus postoperative chemotherapy with fluorouracil, leucovorin calcium, doxorubicin, and cyclophosphamides/granulocyte colony-stimulating factor for stage II breast cancer for both RFS and OS.
Figures

References
-
- American Cancer Society. Breast Cancer Facts and Figures. 2017-2018:1–41.
-
- Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann. Oncol 1999; 10(1):47–52. - PubMed
-
- Danforth DN Jr., Cowan K, Altemus R, et al. Preoperative FLAC/granulocyte-colony-stimulating factor chemotherapy for stage II breast cancer: a prospective randomized trial. Ann. Surg. Oncol 2003; 10(6):635–644. - PubMed
-
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003; 97(11):2869–79. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

