Protective effect of cilastatin against diclofenac-induced nephrotoxicity through interaction with diclofenac acyl glucuronide via organic anion transporters
- PMID: 32000294
- PMCID: PMC7161545
- DOI: 10.1111/bph.14957
Protective effect of cilastatin against diclofenac-induced nephrotoxicity through interaction with diclofenac acyl glucuronide via organic anion transporters
Abstract
Background and purpose: Diclofenac is a widely used nonsteroidal anti-inflammatory drug. However, adverse effects in the kidney limit its clinical application. The present study was aimed to evaluate the potential effect of cilastatin on diclofenac-induced acute kidney injury and to clarify the potential roles of renal organic anion transporters (OATs) in the drug-drug interaction between cilastatin and diclofenac.
Experimental approach: The effect of cilastatin was evaluated in diclofenac-induced acute kidney injury in mice. Human OAT1/3-transfected HEK293 cells and renal primary proximal tubule cells (RPTCs) were used to investigate OAT1/3-mediated transport and the cytotoxicity of diclofenac.
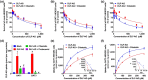
Key results: Cilastatin treatment decreased the pathological changes, renal dysfunction and elevated renal levels of oxidation products, cytokine production and apoptosis induced by diclofenac in mice. Moreover, cilastatin increased the plasma concentration and decreased the renal distribution of diclofenac and its glucuronide metabolite, diclofenac acyl glucuronide (DLF-AG). Similarly, cilastatin inhibited cytotoxicity and mitochondrial damage in RPTCs but did not change the intracellular accumulation of diclofenac. DLF-AG but not diclofenac exhibited OAT-dependent cytotoxicity and was identified as an OAT1/3 substrate. Cilastatin inhibited the intracellular accumulation and decreased the cytotoxicity of DLF-AG in RPTCs.
Conclusion and implications: Cilastatin alleviated diclofenac-induced acute kidney injury in mice by restoring the redox balance, suppressing inflammation, and reducing apoptosis. Cilastatin inhibited OATs and decreased the renal distribution of diclofenac and DLF-AG, which further ameliorated the diclofenac-induced nephrotoxicity in mice. Cilastatin can be potentially used in the clinic as a therapeutic agent to alleviate the adverse renal reaction to diclofenac.
© 2020 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

